Abstract
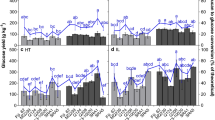
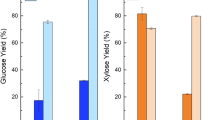
Maximum yield from any cellulosic bioenergy crop is largely dependent upon total dry weight at harvest and process-specific bioconversion rates. Using enzymatic hydrolysis rate as a bioconversion metric, we have investigated the relationship between the biomass crystallinity index (CI) and hydrolysis yield potential (HYP) among ∼20 Sorghum bicolor varieties grown in two environments. The comparison of HYP to CI revealed a significant negative correlation in both environments indicating that high cellulose crystallinity in sorghum can have an impact on conversion yield. Interestingly, no correlation was seen between CI and HYP after pretreatment. Compositional analysis revealed a significant positive correlation between lignin content and CI, as well as a significant negative correlation between lignin content and HYP. Additionally, CI and HYP were found to be significantly correlated only after 24 h of hydrolysis. These results suggest that when a sorghum cultivar is being considered for industrial scale production, the inclusion of cellulose crystallinity should be factored into the decision along with total biomass yield and lignin composition.







Similar content being viewed by others
Abbreviations
- HYP:
-
Hydrolysis yield potential
- CI:
-
Crystallinity index
References
Bak JS, Ko JK, Han YH, Lee BC, Choi I, Kim KH (2009) Improved enzymatic hydrolysis yield of rice straw using electron beam irradiation pretreatment. Bioresour Technol 100:1285–1290
Bansal P, Hall M, Realff MJ, Lee JH, Bommarius AS (2010) Multivariate statistical analysis of X-ray data from cellulose: a new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour Technol 101(12)
Billa E, Koullas DP, Monties B, Koukiosa EG (1997) Structure and composition of sweet sorghum stalk components. Ind Crop Prod 6(3–4):297–302
Casler MD, Hatfield RD (2006) Cell wall composition of smooth bromegrass plants selected by divergent fiber concentration. J Agric Food Chem 54:8206–8211
Chang VS, Holtzapple MT (2000) Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol 84–86:5–37
Ciolacu D, Kovac J, Kokol V (2010) The effect of the cellulose-binding domain from Clostridium cellulovorans on the supramolecular structure of cellulose fibers. Carbohydr Res 345:621–630
Corredor DY, Salazar JM, Hohn KL, Bean S, Bean B, Wang D (2009) Evaluation and characterization of forage sorghum as feedstock for fermentable sugar production. Appl Biochem Biotechnol 158(1):164–179
Coward-Kelly G, Aiello-Mazzari C, Kim S, Granda C, Holtzapple MT (2003) Suggested improvements to the standard filter paper assay used to measure cellulase activity. Biotechnol Bioeng 82(6):745–749
Das KC, Singh K, Bibens B, Hilten R, Baker SA, Greene WD et al (2011) Pyrolysis characteristics of forest residues obtained from different harvesting methods. Appl Eng Agric 27(1):107–113
Edme S, Comstock J, Miller J, Tai P (2005) Determination of DNA content and genome size in sugarcane. J Am Soc Sugar Cane Technol 25:1–16
Esteghlalian AR, Bilodeau M, Mansfield SD, Saddler JN (2001) Do enzymatic hydrolyzability and Simons’ stain reflect the changes in the accessibility of lignocellulosic substrates to cellulase enzymes? Biotechnol Prog 17:1049–1054
Hall M, Bansal P, Lee JH, Realff MJ, Bommarius AS (2010) Cellulose crystallinity—a key predictor of the enzymatic hydrolysis rate. FEBS J 277:1571–1582
Harris D, DeBolt S (2008) Relative crystallinity of plant biomass: studies on assembly, adaptation and acclimation. PLoS One 3(8)
Heaton EA, Flavell RB, Mascia PN, Thomas SR, Dohleman FG, Long SP (2008) Herbaceous energy crop development: recent progress and future prospects. Curr Opin Biotechnol 19(3):202–209
Jahan MS, Mun SP (2005) Effect of tree age on the cellulose structure of Nalita wood (Trema orientalis). Wood Sci Technol 39:367–373
Jung HJG, Ni WT (1998) Lignification of plant cell walls: impact of genetic manipulation. Proc Natl Acad Sci U S A 95(22):12742–12743
Kim S, Holtzapple MT (2006) Delignification kinetics of corn stover in lime pretreatment. Bioresour Technol 97(5):778–785
Kim S, Holtzapple MT (2006) Effect of structural features on enzyme digestibility of corn stover. Bioresour Technol 97:583–591
Kim TH, Kim JS, Sunwoo C, Lee YY (2003) Pretreatment of corn stover by aqueous ammonia. Bioresour Technol 90(1):39–47
Kim TH, Lee YY (2005) Pretreatment of corn stover by soaking in aqueous ammonia. Appl Biochem Biotechnol 124(1–3):1119–1131
Lema M, Felix A, Salako S, Bishnoi U (2000) Nutrient content and in vitro dry matter digestibility of silages made from various grain sorghum and sweet sorghum cultivars. J Sustain Agric 17(1):55–70
Li CL, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M et al (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101(13):4900–4906
Liu L, Sun JS, Li M, Wang SH, Pei HS, Zhang JS (2009) Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour Technol 100(23):5853–5858
Liu RG, Yu H, Huang Y (2005) Structure and morphology of cellulose in wheat straw. Cellulose 12(1):25–34
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Murray S, Rooney W, Hamblin M, Mitchell S, Kresovich S (2009) Sweet sorghum genetic diversity and association mapping for brix and height. Plant Genome 2(1):48–62
Newman RH (2004) Homogeneity in cellulose crystallinity between samples of Pinus radiata wood. Holzforschung 58:91–96
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H et al (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457(7229):551–556
Piedade MTF, Junk WJ, Long SP (1991) The productivity of the C4 grass Echinochloa polystachya on the Amazon floodplain. Ecology 72(4):1456–1463
Rayburn AL, Crawford J, Rayburn CM, Juvik JA (2009) Genome size of three Miscanthus species. Plant Mole Biol Report 27(2):184–188
Reddy N, Yang YQ (2007) Structure and properties of natural cellulose fibers obtained from sorghum leaves and stems. J Agric Food Chem 55(14):5569–5574
Sarath G, Mitchell RB, Sattler SE, Funnell D, Pedersen JF, Graybosch RA et al (2008) Opportunities and roadblocks in utilizing forages and small grains for liquid fuels. J Ind Microbiol Biotechnol 35(5):343–354
Schmer MR, Vogel KP, Mitchell RB, Perrin RK (2008) Net energy of cellulosic ethanol from switchgrass. Proc Natl Acad Sci U S A 105(2):464–469
Schnable PS, Ware D, Fulton RS, Stein JC, Wei FS, Pasternak S et al (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326(5956):1112–1115
Segal L, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794
Selig M, Weiss N, Ji Y (2008) Enzymatic saccharification of lignocellulosic biomass: laboratory analytical procedure (LAP). NREL, Golden, CO
Van Soest PJ, Wine RH (1967) Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J Assoc Off Anal Chem 50:50–55
Vandenbrink JP, Delgado MP, Frederick JR, Feltus FA (2010) A sorghum diversity panel biofuel feedstock screen for genotypes with high hydrolysis yield potential. Ind Crop Prod 31(3):444–448
Wang M (2005) Energy and Greenhouse gas emissions impact of fuel ethanol. In: NGCA Renewable Fuels Forum. Washington, DC, 2005
Westcott PC (2007) Ethanol expansion in the United States: how will the agricultural sector adjust? In: USDA (ed.) USDA Economic Research Service, Denver, CO
Xiao Z, Gao P, Qu Y, Wang T (2001) Cellulose-binding domain of endoglucanase III from Trichoderma reesei disrupting the structure of cellulose. Biotechnol Lett 23:711–715
Yoshida M, Liu Y, Uchida S, Kawarada K, Ukagami Y, Ichinose H et al (2008) Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci Biotechnol Biochem 72(3):805–810
Zhan X, Wang D, Tuinstra MR, Bean S, Seib PA, Sun XS (2003) Ethanol and lactic acid production as affected by sorghum genotype and location. Ind Crop Prod 18:245–255
Acknowledgments
This research was supported in part by the Clemson Experiment Station project #SC-1700381 to FAF and assigned technical contribution no. 5906. We would like to thank Dr. Sarah Harcum and Dr. Don Vanderveer for the gracious use of their equipment.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Rights and permissions
About this article
Cite this article
Vandenbrink, J.P., Hilten, R.N., Das, K.C. et al. Analysis of Crystallinity Index and Hydrolysis Rates in the Bioenergy Crop Sorghum bicolor . Bioenerg. Res. 5, 387–397 (2012). https://doi.org/10.1007/s12155-011-9146-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-011-9146-2