Abstract
Background and Purpose
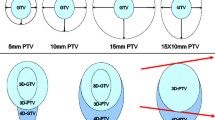
To quantify tumor volume coverage and excess normal tissue coverage using margin expansions of mobile target internal target volumes (ITVs) in the lung.
Materials and methods
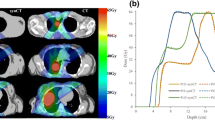
FDG-PET list-mode data were acquired for four spheres ranging from 1 to 4 cm as they underwent 1D motion based on four patient breathing trajectories. Both ungated PET images and PET maximum intensity projections (PET-MIPs) were examined. Amplitude-based gating was performed on sequential list-mode files of varying signal-to-background ratios to generate PET-MIPs. ITVs were first post-processed using either a Gaussian filter or a custom two-step module, and then segmented by applying a gradient-based watershed algorithm. Uniform and non-uniform 1 mm margins were added to segmented ITVs until complete target coverage was achieved.
Results
PET-MIPs required smaller uniform margins (4.7 vs. 11.3 mm) than ungated PET, with correspondingly smaller over-coverage volumes (OCVs). Non-uniform margins consistently resulted in smaller OCVs when compared to uniform margins. PET-MIPs and ungated PET had comparable OCVs with non-uniform margins, but PET-MIPs required smaller longitudinal margins (4.7 vs. 8.5 mm). Non-uniform margins were independent of sphere size.
Conclusions
Gated PET-MIP images and non-uniform margins result in more accurate ITV delineation while reducing normal tissue coverage.






Similar content being viewed by others
References
Chang JY, Dong L, Liu H, Starkschall G, Balter P, Mohan R, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008;3(2):177–86.
Lievens Y, Nulens A, Gaber MA, Defraene G, De Wever W, Stroobants S, et al. Intensity-modulated radiotherapy for locally advanced non-small-cell lung cancer: a dose-escalation planning study. Int J Radiat Oncol Biol Phys. 2011;80(1):306–13.
Liao ZX, Komaki RR, Thames HD Jr, Liu HH, Tucker SL, Mohan R, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(3):775–81.
Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33(10):3874–900.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96.
Bradley JD, Moughan J, Graham MV, Byhardt R, Govindan R, Fowler J, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys. 2010;77(2):367–72.
Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677–82.
Park SJ, Ionascu D, Killoran J, Mamede M, Gerbaudo VH, Chin L, et al. Evaluation of the combined effects of target size, respiratory motion and background activity on 3D and 4D PET/CT images. Phys Med Biol. 2008;53(13):3661–79.
Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59(1):78–86.
Bettinardi V, Picchio M, Di Muzio N, Gianolli L, Gilardi MC, Messa C. Detection and compensation of organ/lesion motion using 4D-PET/CT respiratory gated acquisition techniques. Radiother Oncol. 2010;96(3):311–6.
Aristophanous M, Berbeco RI, Killoran JH, Yap JT, Sher DJ, Allen AM, et al. Clinical utility of 4D FDG-PET/CT scans in radiation treatment planning. Int J Radiat Oncol Biol Phys. 2012;82(1):e99–105.
Hatt M, Maitre AL, Wallach D, Fayad H, Visvikis D. Comparison of different methods of incorporating respiratory motion for lung cancer tumor volume delineation on PET images: a simulation study. Phys Med Biol. 2012;57(22):7409–30.
Ezhil M, Vedam S, Balter P, Choi B, Mirkovic D, Starkschall G, et al. Determination of patient-specific internal gross tumor volumes for lung cancer using four-dimensional computed tomography. Radiat Oncol. 2009;4:4.
Bradley JD, Nofal AN, El Naqa IM, Lu W, Liu J, Hubenschmidt J, et al. Comparison of helical, maximum intensity projection (MIP), and averaged intensity (AI) 4D CT imaging for stereotactic body radiation therapy (SBRT) planning in lung cancer. Radiother Oncol. 2006;81(3):264–8.
Underberg RW, Lagerwaard FJ, Slotman BJ, Cuijpers JP, Senan S. Use of maximum intensity projections (MIP) for target volume generation in 4DCT scans for lung cancer. Int J Radiat Oncol Biol Phys. 2005;63(1):253–60.
Lamb JM, Robinson C, Bradley J, Laforest R, Dehdashti F, White BM, et al. Generating lung tumor internal target volumes from 4D-PET maximum intensity projections. Med Phys. 2011;38(10):5732–7.
Callahan J, Kron T, Schneider-Kolsky M, Dunn L, Thompson M, Siva S, et al. Validation of a 4D-PET maximum intensity projection for delineation of an internal target volume. Int J Radiat Oncol Biol Phys. 2003;86(4):749–54.
Stroom JC, Heijmen BJ. Geometrical uncertainties, radiotherapy planning margins, and the ICRU-62 report. Radiother Oncol. 2002;64(1):75–83.
Geets X, Lee JA, Bol A, Lonneux M, Gregoire V. A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imaging. 2007;34(9):1427–38.
Janssen MH, Aerts HJ, Ollers MC, Bosmans G, Lee JA, Buijsen J, et al. Tumor delineation based on time-activity curve differences assessed with dynamic fluorodeoxyglucose positron emission tomography-computed tomography in rectal cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(2):456–65.
Roels S, Slagmolen P, Nuyts J, Lee JA, Loeckx D, Maes F, et al. Biological image-guided radiotherapy in rectal cancer: challenges and pitfalls. Int J Radiat Oncol Biol Phys. 2009;75(3):782–90.
Hofheinz F, Langner J, Beuthien-Baumann B, Oehme L, Steinbach J, Kotzerke J, et al. Suitability of bilateral filtering for edge-preserving noise reduction in PET. EJNMMI Res. 2011;1(1):23.
Fish DA, Brinicombe AM, Pike ER, Walker JG. Blind deconvolution by means of the Richardson-Lucy algorithm. Journal of the Optical Society of America A. 1995;12(1):58–65.
Lucignani G, Paganelli G, Bombardieri E. The use of standardized uptake values for assessing FDG uptake with PET in oncology: a clinical perspective. Nucl Med Commun. 2004;25(7):651–6.
Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45(9):1431–4.
Meyer F. Topographic distance and watershed lines. Sig Process. 1994;38(1):113–25.
Cheebsumon P, Yaqub M, van Velden FH, Hoekstra OS, Lammertsma AA, Boellaard R. Impact of [(1)(8)F]FDG PET imaging parameters on automatic tumour delineation: need for improved tumour delineation methodology. Eur J Nucl Med Mol Imaging. 2011;38(12):2136–44.
Riddell C, Brigger P, Carson RE, Bacharach SL. The watershed algorithm: a method to segment noisy PET transmission images. IEEE Trans Nucl Sci. 1999;46(3):713–9.
Yang D, Brame S, El Naqa I, Aditya A, Wu Y, Goddu SM, et al. Technical note: DIRART–A software suite for deformable image registration and adaptive radiotherapy research. Med Phys. 2011;38(1):67–77.
Teo BK, Seo Y, Bacharach SL, Carrasquillo JA, Libutti SK, Shukla H, et al. Partial-volume correction in PET: validation of an iterative postreconstruction method with phantom and patient data. J Nucl Med. 2007;48(5):802–10.
Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, Lebesque JV, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53(4):822–34.
Jani SS, Robinson CG, Dahlbom M, White BM, Thomas DH, Gaudio S, et al. A comparison of amplitude-based and phase-based positron emission tomography gating algorithms for segmentation of internal target volumes of tumors subject to respiratory motion. Int J Radiat Oncol Biol Phys. 2013;87(3):562–9.
Bettinardi V, Rapisarda E, Gilardi MC. Number of partitions (gates) needed to obtain motion-free images in a respiratory gated 4D-PET/CT study as a function of the lesion size and motion displacement. Med Phys. 2009;36(12):5547–58.
Dawood M, Buther F, Stegger L, Jiang X, Schober O, Schafers M, et al. Optimal number of respiratory gates in positron emission tomography: a cardiac patient study. Med Phys. 2009;36(5):1775–84.
Davies SC, Hill AL, Holmes RB, Halliwell M, Jackson PC. Ultrasound quantitation of respiratory organ motion in the upper abdomen. Br J Radiol. 1994;67(803):1096–102.
Korin HW, Ehman RL, Riederer SJ, Felmlee JP, Grimm RC. Respiratory kinematics of the upper abdominal organs: a quantitative study. Magn Reson Med. 1992;23(1):172–8.
Acknowledgments
The authors wish to thank Judd Jones, Ph.D., for providing us with user-friendly stand-alone PET reconstruction modules.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jani, S.S., Lamb, J.M., White, B.M. et al. Assessing margin expansions of internal target volumes in 3D and 4D PET: a phantom study. Ann Nucl Med 29, 100–109 (2015). https://doi.org/10.1007/s12149-014-0914-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-014-0914-x