Abstract
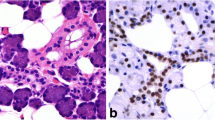
Basal cell adenomas and basal cell adenocarcinomas show marked histomorphologic similarity and are separated microscopically primarily by the invasive characteristics of the adenocarcinomas. We wished to explore potential differences in the expression of epithelial–mesenchymal transition associated proteins in these two tumor types. A tissue microarray was constructed utilizing 29 basal cell adenomas and 16 basal cell adenocarcinomas. Immunohistochemical expression of E-cadherin, beta-catenin, Twist 1 and vimentin were investigated. Both tumors expressed all proteins in a relatively similar manner. Nuclear beta-catenin was essentially limited to the abluminal cell populations in both tumor types. E-cadherin was limited largely to luminal locations but was more prevalent in the adenocarcinomas as compared to the adenomas. Primarily abluminal expression for vimentin was seen, sometimes present in an apical dot-like pattern. Distinct populations of cellular expression of these four markers of epithelial mesenchymal transition were present but were similar in locations in both tumors with no patterns discerned to separate basal cell adenoma from basal cell adenocarcinoma. Given these findings, the mechanisms by which basal cell adenocarcinoma is able to invade while its counterpart, basal cell adenoma can not, may be more complex than in other tumor types.





Similar content being viewed by others
References
Wilson T, Robinson RA. Basal cell adenoma and basal cell adenocarcinoma of the salivary glands: a clinicopathological of seventy tumors with comparison of morphologic features and growth control indices. Head Neck Pathol. 2015;9(2):205–13.
Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233(3):706–20.
Montgomery E, Folpe AL. The diagnostic value of beta-catenin immunohistochemistry. Adv Anat Pathol. 2005;12(6):350–6.
Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49(4):287–92.
Nijkamp MM, Span PN, Hoogsteen IJ, van der Kogel AJ, Kaanders JH, Bussink J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiother Oncol. 2011;99(3):344–8.
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–804.
Economopoulou P, Hanby A, Odell EW. Expression of E-cadherin, cellular differentiation and polarity in epithelial salivary neoplasms. Oral Oncol. 2000;36(6):515–8.
Furuse C, Cury PR, Altemani A, dos Santos Pintos D Jr, de Araujo NS, de Araujo VC. Beta-catenin and E-cadherin expression in salivary gland tumors. Int J Surg Pathol. 2006;12(3):212–7.
Andreadis D, Epivatianos A, Mireas G, et al. Immunohistochemical detection of E-cadherin in certain types of salivary gland tumours. J Laryngol Otol. 2006;120(4):298–304.
Prabhu S, Kaveri H, Rekha K. Benign, malignant salivary gland tumors: comparison of immunohistochemical expression of e-cadherin. Oral Oncol. 2009;45(7):594–9.
Toshitaka N. Immunohistochemical analysis of salivary gland tumors: applications for surgical pathology practice. Acta Histochem Cytochem. 2012;45(5):269–82.
Kawahara A, Harada H, Abe H, Yamaguchi T, Taira T, Nakashima K, et al. Nuclear B-catenin expression in basal cell adenomas of salivary gland. J Oral Pathol Med. 2011;40(6):460–6.
Chandrashekar C, Angadi PV, Krishnapillai R. B-catenin expression in benign and malignant salivary gland tumors. Int J Surg Pathol. 2011;19(4):433–40.
Mantesso A, Loducca SV, Jaeger RG, Decio SP, Araujo VC. Analysis of epithelial–myoepithelial carcinoma based on the establishment of a novel cell line. Oral Oncol. 2003;39:453–8.
Lill C, Schneider S, Seemann R, Kadletz L, Aumayr K, Ghanim B, et al. Correlation of B-catenin, but not PIN1 and cyclin D1, overexpression with disease-free and overall survival in patients with cancer of the parotid gland. Head Neck. 2015;37(1):30–6.
Shieh YS, Change LC, Chiu KC, Wu CW, Lee HS. Cadherin and catenin expression in mucoepidermoid carcinoma: correlation with histopathologic grade, clinical stage and patient outcome. J Oral Pathol Med. 2003;32(5):297–304.
Ansieau S, Bastid J, Doreau A, Morel A-P, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89.
Zhou C, Liu J, Tang Y, Zhu G, Zheng M, Jiang J, et al. Coexpression of hypoxia-inducible factor-2α, TWIST2, and SIP1 may correlate with invasion and metastasis of salivary adenoid cystic carcinoma. J Oral Pathol Med. 2012;41(5):424–31.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39.
Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367(2):235–41.
Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol. 2014;50(1):1–6.
Acknowledgments
This work was funded by the Department of Pathology, University of Iowa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Tesdahl, B.A., Wilson, T.C., Hoffman, H.T. et al. Epithelial–Mesenchymal Transition Protein Expression in Basal Cell Adenomas and Basal Cell Adenocarcinomas. Head and Neck Pathol 10, 176–181 (2016). https://doi.org/10.1007/s12105-015-0657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-015-0657-6