Abstract
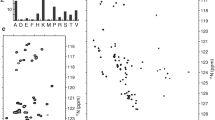
Human emerin is an inner nuclear membrane protein involved in the response of the nucleus to mechanical stress. It contributes to the physical connection between the cytoskeleton and the nucleoskeleton. It is also involved in chromatin organization. Its N-terminal region is nucleoplasmic and comprises a globular LEM domain from residue 1 to residue 43. The three-dimensional structure of this LEM domain in complex with the chromatin BAF protein was solved from NMR data. Apart from the LEM domain, the nucleoplasmic region of emerin, from residue 44 to residue 221, is predicted to be intrinsically disordered. Mutations in this region impair binding to several emerin partners as lamin A, actin or HDAC3. However the molecular details of these recognition defects are unknown. Here we report 1H, 15N, 13CO, 13Cα and 13Cβ NMR chemical shift assignments of the emerin fragment from residue 67 to residue 170, which is sufficient for nuclear localization and involved in lamin A binding. Chemical shift analysis confirms that this fragment is intrinsically disordered in 0 and 8 M urea.


Similar content being viewed by others
References
Berk JM, Tifft KE, Wilson KL (2013) The nuclear envelope LEM-domain protein emerin. Nucleus 4:298–314. doi:10.4161/nucl.25751
Bertrand AT et al (2014) Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J Cell Sci 127:2873–2884. doi:10.1242/jcs.144907
Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D (1994) Identification of a novel X-linked gene responsible for Emery–Dreifuss muscular dystrophy. Nat Genet 8:323–327. doi:10.1038/ng1294-323
Cai M, Huang Y, Suh JY, Louis JM, Ghirlando R, Craigie R, Clore GM (2007) Solution NMR structure of the barrier-to-autointegration factor-Emerin complex. J Biol Chem 282:14525–14535. doi:10.1074/jbc.M700576200
Clements L, Manilal S, Love DR, Morris GE (2000) Direct interaction between emerin and lamin A. Biochem Biophys Res Commun 267:709–714. doi:10.1006/bbrc.1999.2023
Ellis JA, Craxton M, Yates JR, Kendrick-Jones J (1998) Aberrant intracellular targeting and cell cycle-dependent phosphorylation of emerin contribute to the Emery-Dreifuss muscular dystrophy phenotype. J Cell Sci 111(Pt 6):781–792
Haque F et al (2006) SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol 26:3738–3751. doi:10.1128/MCB.26.10.3738-3751.2006
Herrada I, Bourgeois B, Samson C, Buendia B, Worman HJ, Zinn-Justin S (2015) Purification and structural analysis of LEM-domain proteins. Methods Enzymol
Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J (2013) Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497:507–511. doi:10.1038/nature12105
Shen Y, Bax A (2010) Prediction of Xaa-Pro peptide bond conformation from sequence and chemical shifts. J Biomol NMR 46:199–204. doi:10.1007/s10858-009-9395-y
Swift J, Discher DE (2014) The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J Cell Sci 127:3005–3015. doi:10.1242/jcs.149203
Tamiola K, Acar B, Mulder FA (2010) Sequence-specific random coil chemical shifts of intrinsically disordered proteins. J Am Chem Soc 132:18000–18003. doi:10.1021/ja105656t
Vranken WF et al (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696. doi:10.1002/prot.20449
Wolff N, Gilquin B, Courchay K, Callebaut I, Worman HJ, Zinn-Justin S (2001) Structural analysis of emerin, an inner nuclear membrane protein mutated in X-linked Emery–Dreifuss muscular dystrophy. FEBS Lett 501:171–176
Acknowledgments
We thank the French Association against Myopathies (AFM) (research Grant No. 17243 to S.Z.J. and fellowship No. 18159 to C.S.) and the Foundation for Medical Research (FRM) (Grant. FDT20140931008 to I.H.) for providing financial support to this project. We also thank the Leibniz-Institut für Molekular Pharmakologie (FMP) for access to the NMR facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Camille Samson, Isaline Herrada have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Samson, C., Herrada, I., Celli, F. et al. 1H, 13C and 15N backbone resonance assignment of the intrinsically disordered region of the nuclear envelope protein emerin. Biomol NMR Assign 10, 179–182 (2016). https://doi.org/10.1007/s12104-015-9662-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-015-9662-7