Abstract
The Fc portion of immunoglobulin G (IgG) recruits complements and its cognate receptors, thereby promoting defensive mechanisms in the humoral immune system. These effector functions critically depend on N-glycosylation at the Fc region, which is therefore regarded as a crucial factor in the design and production of therapeutic antibodies. NMR spectroscopy plays a unique role in the characterization of conformational dynamics and intermolecular interactions of IgG-Fc in solutions. Here, we report NMR assignments of the glycosylated Fc fragment (Mr 53 kDa), cleaved from a chimeric antibody with human IgG1 constant regions, which was produced in Chinese hamster ovary cells with uniform 13C- and 15N-labeling.
Similar content being viewed by others
Biological context
Immunoglobulin G (IgG) is a multifunctional glycoprotein composed of an Fc region and two Fab regions, which are connected through the hinge region (Yamaguchi et al. 2007). The Fab regions recognize and capture specific antigens, while the Fc region recruits complements and its cognate receptors, Fcγ receptors (FcγRs), and offers acceptor sites for bacterial proteins including protein A and protein G. The Fc region has a homodimeric structure comprising the C-terminal halves of the heavy chains, each composed of the CH2 and CH3 domains. The CH2 domain possesses a conserved N-glycosylation site, Asn297, at which a biantennary complex-type oligosaccharide is expressed with microheterogenieties characterized by the presence and absence of the non-reducing terminal galactose, fucose, sialic acid, and bisecting N-acetylglucosamine residues.
The effector function of IgG critically depends on N-glycosylation in the Fc region. The outer carbohydrate moieties govern the structural integrity of the FcγR-binding site of IgG, while the core fucosylation impairs antibody-dependent cellular cytotoxicity because of its negative steric effect against IgG interaction with FcγRIII (Ferrara et al. 2011; Krapp et al. 2003; Mizushima et al. 2011; Yamaguchi et al. 2006). Hence, the Fc glycoforms are now considered as a crucial factor in the design and production of therapeutic antibodies in biopharmaceutical fields (Berkowitz et al. 2012; Jiang et al. 2011).
NMR spectroscopy offers unique tools for characterizing the conformational dynamics and intermolecular interactions of IgG-Fc in solution (Kato et al. 1991a, 1993a, 1995; Kim et al. 1994a; Latypov et al. 2012). We developed protocols for uniform and amino acid-selective stable isotope labeling of an IgG glycoprotein and its functional fragments, using mammalian expression systems (Kato et al. 2010; Yamaguchi and Kato 2010). Based on partially (approximately 66 %) achieved spectral assignments (Yamaguchi et al. 2006), we previously reported NMR analytical results to characterize the N-glycosylation-dependent conformational changes of human IgG1-Fc and its interaction with a specific RNA aptamer (Matsumiya et al. 2007; Miyakawa et al. 2008; Yamaguchi et al. 2006).
In an extension of these studies, we herein report NMR assignments of the glycosylated version of Fc fragment (Mr 53 kDa) cleaved from a chimeric antibody with human IgG1 constant regions that was expressed by Chinese hamster ovary (CHO) cells with uniform 13C- and 15N-labeling.
Methods and experiments
The CHO/DG44 cell line (Urlaub1980) was kindly provided by Dr. Lawrence Chasin (Columbia University, NY). An anti-CCR4 chimeric antibody (designated KM3060), with human IgG1/κ constant regions, was produced in a CHO cell line as described previously (Yamaguchi and Kato 2010; Yamaguchi et al. 2006). The CHO cells were cultivated using the Nissui NYSF 404 medium supplemented with 2 % dialyzed fetal bovine serum. At the final stage of cell culture, the medium was replaced with an isotopically labeled one with 2 % dialyzed fetal bovine serum. Uniformly 15N/13C-labeled IgG1 was prepared using a modified Nissui NYSF 404 medium (supplemented with 2 % dialyzed fetal bovine serum) in which glucose, sodium pyruvate, succinic acid, and amino acids were replaced by 1 g/L [15N/13C]algal amino acid mixture, 2 g/L D-[13C6]glucose, 110 mg/L [13C3]pyruvic acid sodium salt, 59 mg/L [13C4]succinic acid, 149 mg/L L-[13C6,15N4]Arg·HCl, 42.5 mg/L L-[13C4,15N2]Asn·H2O, 24 mg/L L-[13C3,15N]Cys, 450 mg/L L-[13C5,15N2]Gln, 17 mg/L L-[13C6,15N3]His·HCl·H2O, 27 mg/L L-[13C9,15N]Tyr, and 7 mg/L L-[13C11,15N2]Trp. Amino acid-selective labeling of IgG1 was performed using the modified Nissui NYSF 404 medium (supplemented with 2 % dialyzed fetal bovine serum) in which selected amino acid components were substituted with isotopically labeled analogs, as described previously (Kato et al. 1991a, b, 1993b; Kim et al. 1994b). After cell growth, the supernatant was purified using an Affi-gel protein A column (GE Healthcare Bio-Sciences), as described previously (Yamaguchi et al. 1995). The Fc fragment of IgG1 was prepared by papain digestion, performed at 37 °C for 12 h in 75 mM sodium phosphate buffer (pH 7.0) containing 75 mM NaCl, and 2 mM EDTA. The protein concentration was 10 mg/ml, and the ratio of papain/IgG1 was 1:50 (w:w). The digestion products were loaded onto an Affi-gel protein A column. To prepare IgG1-Fc exhibiting a homogeneous N-glycan, GlcNAcβ1-2Manα1-3(GlcNAcβ1-2Manα1-6)Manβ1-4GlcNAcβ1-4(Fucα1-6)GlcNAc, the Fc fragment was treated with a recombinant Streptococcus pneumoniae β1,4-galactosidase (CALBIOCHEM), according to the literature (Yamaguchi et al. 2006).
For NMR measurements, the Fc fragment was dissolved in 0.5 ml of 5 mM sodium phosphate buffer (pH 6.0) containing 50 mM NaCl and 10 % (v/v) D2O. NMR spectra were acquired at 42 or 52 °C using DMX500 (Bruker BioSpin), AVANCE800 (Bruker BioSpin), and ECA-920 (JEOL) spectrometers. Chemical shifts of 1H were referenced to DSS (0 ppm), and 13C and 15N chemical shifts were referenced indirectly using the gyromagnetic ratios of 13C, 15N, and 1H (γ 13C/γ 1H = 0.25144952; γ 15N/γ 1H = 0.10132905).
Backbone resonance assignments were made on the basis of 2D 1H–15N HSQC spectral data of uniformly or selectively 13C/15N-labeled IgG1-Fc, and 3D spectral data obtained with the following experiments: HNCA, HNCO, HN(CA)CO, CBCA(CO)NH, and HNCACB. All NMR data were processed using NMRPipe software (Delaglio et al. 1995), and analyzed with SPARKY (Goddard and Kneller 1993) and CcpNmr (Vranken et al. 2005) software.
Assignments and data deposition
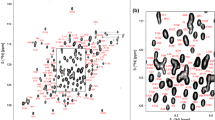
Figure 1 shows the 1H–15N HSQC spectrum of human IgG1-Fc. Although the use of a mammalian expression system is mandatory for preparing antibodies with physiological glycosylation, uniform deuteration of the glycoprotein is not facile in such a system (Liu et al. 2007). Hence, we established spectral assignments based on the triple resonance spectral dataset recorded at a higher temperature, i.e. 52 °C, complemented with HSQC spectral data obtained by amino acid-selective 13C/15N-labeling. Chemical shift assignments were made for protein backbone resonances: Cα (99 %), Cβ (84 %), CO (80 %), HN (99 %), and N (99 %) (except for N of prolines). The spectral assignments at lower temperatures could be extrapolated by observing progressive spectral changes, depending on temperature, as exemplified by the spectrum at 42 °C (Supplemental Fig.1). The present spectral assignments indicate that a cluster of amino acid residues in the vicinity of the N-glycans, i.e. Gln295-Thr299 exhibit significant chemical shift differences in comparison with the previously reported assignments of human Fc produced in Escherichia coli (Liu et al. 2007).
The assignments for the 1H, 13C, and 15N backbone resonances of human IgG1-Fc have been deposited in the BioMagResBank database (http://www.bmrb.wisc.edu) under the accession number 25224.
References
Berkowitz SA, Engen JR, Mazzeo JR, Jones GB (2012) Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov 11:527–540
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Ferrara C, Grau S, Jäger C, Sondermann P, Brünke P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umaña P, Benz J (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci USA 108:12669–12674
Goddard T, Kneller D (1993) SPARKY 3. University of California, San Francisco
Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M (2011) Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov 10:101–111
Kato K, Matsunaga C, Igarashi T, Kim H, Odaka A, Shimada I, Arata Y (1991a) Complete assignment of the methionyl carbonyl carbon resonances in switch variant anti-dansyl antibodies labeled with [1-13C]methionine. Biochemistry 30:270–278
Kato K, Matsunaga C, Odaka A, Yamato S, Takaha W, Shimada I, Arata Y (1991b) Carbon-13 NMR study of switch variant anti-dansyl antibodies: antigen binding and domain-domain interactions. Biochemistry 30:6604–6610
Kato K, Gouda H, Takaha W, Yoshino A, Matsunaga C, Arata Y (1993a) 13C NMR study of the mode of interaction in solution of the B fragment of staphylococcal protein A and the Fc fragments of mouse immunoglobulin G. FEBS Lett 328:49–54
Kato K, Gouda H, Takaha W, Yoshino A, Matsunaga C, Arata Y (1993b) 13C NMR study of the mode of interaction in solution of the B fragment of staphylococcal protein A and the Fc fragments of mouse immunoglobulin G. FEBS Lett 328:49–54
Kato K, Lian LY, Barsukov IL, Derrick JP, Kim H, Tanaka R, Yoshino A, Shiraishi M, Shimada I, Arata Y et al (1995) Model for the complex between protein G and an antibody Fc fragment in solution. Structure 3:79–85
Kato K, Yamaguchi Y, Arata Y (2010) Stable-isotope-assisted NMR approaches to glycoproteins using immunoglobulin G as a model system. Prog Nucl Magn Reson Spectrosc 56:346–359
Kim H, Matsunaga C, Yoshino A, Kato K, Arata Y (1994a) Dynamical structure of the hinge region of immunoglobulin G as studied by 13C nuclear magnetic resonance spectroscopy. J Mol Biol 236:300–309
Kim H, Matsunaga C, Yoshino A, Kato K, Arata Y (1994b) Dynamical structure of the hinge region of immunoglobulin G as studied by 13C nuclear magnetic resonance spectroscopy. J Mol Biol 236:300–309
Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P (2003) Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol 325:979–989
Latypov RF, Hogan S, Lau H, Gadgil H, Liu D (2012) Elucidation of acid-induced unfolding and aggregation of human immunoglobulin IgG1 and IgG2 Fc. J Biol Chem 287:1381–1396
Liu D, Cocco MJ, Rosenfied R, Lewis JK, Ren D, Li L, Remmele RL Jr, Brems DN (2007) Assignment of backbone 1H, 13C and 15N resonances of human IgG1 Fc (51.4 kDa). Biomol NMR Assign 1:233–235
Matsumiya S, Yamaguchi Y, Saito J, Nagano M, Sasakawa H, Otaki S, Satoh M, Shitara K, Kato K (2007) Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol 368:767–779
Miyakawa S, Nomura Y, Sakamoto T, Yamaguchi Y, Kato K, Yamazaki S, Nakamura Y (2008) Structural and molecular basis for hyperspecificity of RNA aptamer to human immunoglobulin G. RNA 14:1154–1163
Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M, Kato K (2011) Structural basis for improved efficacy of therapeutic antibodies upon defucosylation of their Fc glycans. Genes Cells 16:1071–1080
Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696
Yamaguchi Y, Kato K (2010) Dynamics and interactions of glycoconjugates probed by stable-isotope-assisted NMR spectroscopy. Methods Enzymol 478:305–322
Yamaguchi Y, Kim H, Kato K, Masuda K, Shimada I, Arata Y (1995) Proteolytic fragmentation with high specificity of mouse immunoglobulin G. Mapping of proteolytic cleavage sites in the hinge region. J Immunol Methods 181:259–267
Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K (2006) Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta 1760:693–700
Yamaguchi Y, Takahashi N, Kato K (2007) Antibody Strucutres. In: Kamerling JP (ed) Comprehensive glycoscience, vol 3. Elsevier, Oxford, pp 745–763
Acknowledgments
We would like to thank Ms. Kumiko Hattori and Ms. Kiyomi Senda (Nagoya City University) for their help with recombinant protein preparation. This work was supported, in part, by the Nanotechnology Platform Project, the Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO), and Grants-in-Aid for Scientific Research (24249002 and 25860053) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hirokazu Yagi and Ying Zhang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Yagi, H., Zhang, Y., Yagi-Utsumi, M. et al. Backbone 1H, 13C, and 15N resonance assignments of the Fc fragment of human immunoglobulin G glycoprotein. Biomol NMR Assign 9, 257–260 (2015). https://doi.org/10.1007/s12104-014-9586-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-014-9586-7