Abstract
Objectives
To determine the prevalence of hypophosphatemia in critically ill children and its association with clinical outcomes; to determine risk factors and mechanism of hypophosphatemia.
Methods
Levels of serum phosphate, phosphate intake, renal phosphate handling indices and blood gases were measured on days 1, 3, 7 and 10 of pediatric intensive care unit (PICU) stay. Hypophosphatemia was defined as any serum phosphorus <3.8 mg/dl for children younger than 2 y and <3.5 mg/dl for children 2 y or older. Renal phosphate loss was assessed using the ratio of tubular maximum reabsorption of phosphate (TmP) to glomerular filtration rate (GFR) [TmP/GFR].
Results
Prevalence of hypophosphatemia was 71.6 % (95 % CI: 64.6–78.6). On adjusted analysis, hypophosphatemia was associated with prolonged PICU length of stay (PICU LOS > 6 d) (adjusted OR: 3.0 [95 % CI: 1.4–6.7; p = 0.005]) but not associated with increased mortality. Renal phosphate threshold was significantly lower on all the days in hypophosphatemic group compared to that of non-hypophosphatemic group. No statistically significant difference in the amount of phosphate intake was seen in both the groups.
Conclusions
Hypophosphatemia is highly prevalent in critically ill children and is associated with prolonged PICU LOS. Increased phosphate loss in urine is one of the mechanism responsible for hypophosphatemia in critically ill children.
Similar content being viewed by others
Introduction
Phosphate is a constituent of various intermediate compounds involved in key physiological processes such as adenosine triphosphate, 2,3-diphosphoglycerate and intracellular chemical messengers (e.g., cyclical adenosine monophosphate, cyclical guanosine monophosphate) [1, 2]. The consequences of hypophosphatemia on various organ systems in critical care setting is attributed to deficiency in intermediate compounds. The possible mechanisms for hypophosphatemia in intensive care setting are decreased absorption, increased renal excretion or internal redistribution of inorganic phosphate due to alkalosis [3]. There have been only a few studies in sick children to assess the prevalence and outcomes of hypophosphatemia but none have attempted to explore the possible mechanism responsible for it [4–6].
Therefore, the present study was conducted to determine the prevalence of hypophosphatemia in sick children and its association with various clinical outcomes. The authors have also tried to explore the possible mechanisms responsible for hypophosphatemia. This will help in increasing understanding of phosphate homeostasis and guide in screening and treatment of hypophosphatemia in Pediatric Intensive Care Unit (PICU).
Material and Methods
This was a prospective, observational, cohort study of patients consecutively admitted to the medical PICU at a tertiary care hospital of India from August 2011 through January 2013. Children with expected abnormality involving phosphate homeostasis like Chronic Kidney Disease (CKD), Acute Kidney Injury (AKI) at the time of admission, refractory rickets secondary to renal tubular acidosis, underlying parathyroid disorder, acute leukemia, readmission, death within 24 h of PICU stay and transfer out to wards within 24 h were exclusion criteria. Ethical approval was obtained from the Institute ethics committee and children were enrolled in the study after written informed parental consent. Baseline demographic and diagnostic data were collected on admission to PICU. Pediatric Index of Mortality score (PIM2) was used to assess the severity of illness [7]. Early morning serum phosphate, urea, creatinine, albumin, calcium, alkaline phosphatase and arterial/venous pH were measured at the time of admission, and on D3, D7 and D10 if child stayed in PICU. Serum Parathyroid hormone (PTH) and vitamin D level were measured at the baseline. Early morning timed urinary/ spot urinary sample was sent for Urine Creatinine and Phosphate estimation on different days to measure the ratio of tubular maximum reabsorption of phosphate (TmP) to glomerular filtration rate (GFR) [TmP/GFR]. This index represents the blood concentration of phosphate, above which kidney excretes most of the phosphate and below which most of it is reabsorbed. Normal TmP/GFR range for children is 2.8–4.4 mg/dl [8]. Walton and Bijvoet normogram was used to calculate TmP/GFR with the help of serum phosphate and Tubular reabsorption of phosphate (TRP). TRP = 100 – Fractional excretion of phosphate. Fractional excretion of phosphate (FePO4) was calculated using the equation: (Urine Phosphate × Serum Creatinine)/(Plasma Phosphate × Urine Creatinine) × 100. Intake of medications known to cause hypophosphatemia: furosemide, steroids, insulin, epinephrine, dopamine, salbutamol, phenytoin, phenobarbitol, valproate, mannitol and acetazolamide were recorded during the study period [9]. Phosphate intakes through enteral or parenteral nutrition were recorded. Further standard follow up and blood investigations were not measured in children who were discharged to the ward within 10 d.
Child was said to be hypophosphatemic if any serum phosphate measurement was <3.8 mg/dl for children younger than 2 y and <3.5 mg/dl for children 2 y or older [4, 10]. Starvation time prior to admission was defined as time in hours for which child was not fed by mouth at the time of admission in PICU. Acute Kidney Injury Network criteria was used for defining AKI based on either serum creatinine criteria or urine output criteria [11]. Inappropriately high renal phosphate excretion was defined as TmP/GFR < 2.8 mg/dl [8]. Malnutrition was defined as weight for age ‘z’ score < −2 for children up to 10 y and BMI for age ‘z’ score < −2 for children above 10 y as per World Health Organization (WHO) growth chart and WHO reference [12]. Duration of PICU stay for >6 d was taken as cutoff to define prolonged PICU stay and duration of ventilatory support for >7 consecutive days was taken as cutoff to define prolonged ventilator support based on duration greater than median duration from previous studies done in this unit [13]. Standard definitions of sepsis, septic shock and Acute Respiratory Distress Syndrome (ARDS) were used [14, 15]. Extubation failure was defined as reintubation within 24 h of planned extubation. As per unit’s policy, a child would be started on enteral feeding once he is hemodynamically stable and in case of prolonged hemodynamic instability, parenteral nutrition would be started which does not have phosphate content in it. No interventions were done for the study purpose alone. However, enteral phosphate supplementation was done in children with serum phosphate <2.0 mg/dl as intravenous phosphate was not available at the time of study.
The study determined the proportion of enrolled children in PICU who developed hypophosphatemia during first 10 d of PICU stay (prevalence). Association of hypophosphatemia on mortality, PICU length of stay (LOS) and duration of mechanical ventilation were studied. Amount of phosphate intake, pH and TmP/GFR were determined both in hypophosphatemic and non-hypophosphatemic group to study the potential mechanism of hypophosphatemia.
Serum phosphate and urinary phosphate were measured by colorimetric method based on the principle of ammonium phosphomolybdate complex (Hitachi Modular P800, Cobas, Roche) autoanalyzer. ABL 800 flex (Radiometer Copenhagen) was used for pH measurement.
Data were analyzed at the end of study period using STATA 11 software (StataCorp, College Station, TX). Categorical data were compared using chi-square test and continuous variables were compared using Mann-Whitney U-test. After bi-variate analysis of all factors for association with outcomes, a multivariate logistic regression of all factors with p value of <0.1 in the bi-variate analysis was performed. P value <0.05 was considered significant.
Results
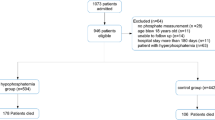
Of the 349 children admitted to the PICU, 162 children were included in the study (Fig. 1). The baseline characteristics of the patients are shown in Table 1.
A total of 444 serum phosphate measurements were carried out with median (range) of the number of phosphate measurements 3 (1, 4) per patient. The total number of measurements performed were 162, 133, 86 and 63 on D1, D3, D7 and D10, respectively. The median (IQR) serum phosphate concentrations on D1, D3, D7 and D10 were 3.7 (2.9, 4.4) mg/dl, 3.2 (2.5, 3.9) mg/dl, 3.6 (2.5, 4.1) mg/dl and 4.0 (3.0, 4.8) mg/dl, respectively.
Hypophosphatemia occurred in 116 patients (71.60 % [95 % CI: 64.6–78.6 %]) during the study period. Prevalence of hypophosphatemia on D1, D3, D7 and D10 was 44.4 %, 63.9 %, 56.9 % and 42.8 % respectively. Most of the children developed hypophosphatemia on day 3. Of the 116 children with hypophosphatemia, 43 children had only single hypophosphatemic value, 42 had 2 values, 18 had 3 values while 13 had all 4 values in the hypophosphatemic range. Of the 90 children who did not have hypophosphatemia on D1, 44 (48.9 %) developed hypophosphatemia subsequently. Fifty-six (34.5 %) children had at least one of the serum phosphate values <2.5 mg/dl whereas 17 (10.5 %) children had at least one of the serum phosphate values <1.5 mg/dl. Eight children received enteral phosphate supplementation during first 10 d of PICU stay.
Association of hypophosphatemia with various outcomes is shown in Table 2. Duration of PICU LOS was greater in hypophosphatemic group [7 (4–17) vs. 4 (2–10); p = 0.001]. On multivariate analysis, hypophosphatemia was associated with prolonged PICU stay (PICU LOS > 6 d) with adjusted odds ratio of 3.0 [95 % CI: 1.4–6.7; p = 0.005]. Besides hypophosphatemia, mechanical ventilation during first 24 h of PICU stay was associated with prolonged PICU LOS (adjusted OR 5.8 [2.4–14.1]; p = 0.001) (Table 3). There was no difference in mortality between the two groups.
Although duration of mechanical ventilation was greater in hypophosphatemic group, no significant association was observed between hypophosphatemia and prolonged ventilator support (ventilator support >7 consecutive days) on adjusted analysis (OR 1.8 [0.7–4.1]; p = 0.19). Extubation failure was observed in 10 children. Out of these 10 children, hypophosphatemia was observed in 9 children. The median (IQR) phosphate values in 10 children who failed extubation during the study period was 3.4 (2.2–3.6) mg/dl as compared to 3.8 (3.3–4.3) in those who did not fail extubation (n = 31); p = 0.04. No association was seen between hypophosphatemia and any specific factors (Table 4).
There was no statistical difference in amount of phosphate intake on most of the days except on D3 where amount of phosphate intake was less in hypophosphatemic group compared to that of non-hypophosphatemic group and was statistically significant (14.4 vs. 24.4 mg/kg, p = 0.04). No significant correlation was seen between amount of phosphate intake and total calorie intake (Table 5).
Median TmP/GFR on all days were lower in hypophosphatemic group compared to non-hypophosphatemic group (Table 5).
There was no difference in parathyroid hormone (PTH) levels between the two groups (Table 5).
Trend towards higher pH value was seen in hypophosphatemic group compared to non-hypophosphatemic group, although it was not statistically significant (Table 5).
Discussion
In this prospective study, hypophosphatemia was common in critically ill children (prevalence 71.6 %) in the first 10 d of admission and more common in initial 3 d. It was independently associated with prolonged PICU LOS but was not associated with increased mortality or increased duration of mechanical ventilation. No specific factors associated with hypophosphatemia could be identified. Increased renal excretion of phosphate was the major mechanism of hypophosphatemia, caused by factors yet unknown. Inability to provide adequate amount of phosphate during the phase of increased excretion through urine may be one of the mechanisms responsible for hypophosphatemia in critical care setting.
The prevalence of hypophosphatemia in this study was slightly higher than previous reports in critically ill children which showed the prevalence of 60 % [4, 5]. This observation may be due to increased frequency of serum phosphate measurement in the index study. However, from all the reports, it is clear that there is higher prevalence of hypophosphatemia in PICU.
This study shows an independent association of hypophosphatemia and prolonged PICU LOS. Similar observation has been reported by Kilic et al. [5]. Many children are admitted in intensive care unit for respiratory support. It has been shown that hypophosphatemia may impair diaphragmatic contractility during acute respiratory failure and may be one of the causes for difficult weaning [16, 17]. There was increased duration of mechanical ventilation in hypophosphatemic group but the authors could not find independent association between hypophosphatemia and prolonged mechanical ventilation as there may be various other causes for prolonged requirement of mechanical ventilation. It is interesting to note that, 10 children had to be reintubated within 24 h of planned extubation and out of them 9 children were hypophosphatemic. Therefore, besides looking for other causes for extubation failure, one should also consider hypophosphatemia as one of the contributing factors if serum phosphate is low.
One of the risk factors for hypophosphatemia is malnutrition [18, 19]; the authors however, could not find association between hypophosphatemia and malnutrition in the present study. The possible reason could be higher frequency of malnourished children in the study population due to higher number of admission of patients with underlying chronic illness, which might have diluted the study population to reach the statistical significance. Sepsis is considered as an another important risk factor for hypophosphatemia [20–22]. Although, greater proportion of septic patients in index study were hypophosphatemic but this observation did not reach statistical significance. Trends towards hypophosphatemia was seen in patients in whom acyclovir and valproate were used. It has been observed that acyclovir causes downregulation of NPT2a transporter and valproate causes Fanconi syndrome like picture, both leading to inadequate tubular reabsorption of phosphate [23, 24].
The novelty of the study is that it looked for the possible causes of hypophosphatemia. Mechanism of hypophosphatemia in critically ill children has not been studied in depth. Literature available from studies done in adults suggests that hypophosphatemia is primarily due to decreased intake, intracellular shift and increased excretion [1, 3]. The amount of phosphate to be supplemented in critical care setting is largely unknown, especially in children. In the present study, authors found trend towards decreased phosphate intake in critically ill children who developed hypophosphatemia although it was not statistically significant. One could think that, decreased amount of calorie intake might be responsible for decreased intake of serum phosphate, however, authors did not find any correlation between calorie intake and total phosphate intake in the present cohort. The possible cause for this observation may be unavailability of parenteral phosphate solution and maybe increased amount of phosphate required to maintain normality. Also no association was found between delay in initiating enteral nutrition and hypophosphatemia. Study by Kilic et al., found association between hypophosphatemia and low phosphate intake through enteral nutrition [5]. The authors, however, believe that low phosphate intake may be one of the mechanism responsible for hypophosphatemia but not the sole cause. It was interesting to note that the patient who developed hypophosphatemia continued to excrete large amount of phosphate in urine not related to PTH level. This observation is similar to the study done in adults by Bech et al. and Srinivasagam et al., where they found that hypophosphatemia in the ICU is commonly associated with renal phosphate loss and not related to phosphaturic hormones like PTH, FGF-23 or calcitonin [25, 26]. To the best of authors’ knowledge, this is the first study to look at renal tubular excretion of phosphate in critically ill children. All these reports suggest that increased loss of phosphate through urine is the major cause of hypophosphatemia in critical illness. And there exists a missing factor which might be responsible for phosphaturic action in critical illness. It has been shown that rise in intracellular pH stimulates phosphofructokinase activity, which in turn, stimulates glycolysis and increases the formation of phosphorylated carbohydrate which leads to extracellular hypophosphatemia [27, 28]. In this study, trend towards increased pH was seen in hypophosphatemic group but it failed to reach the statistical significance.
The strength of the study is that it was a prospective study and size of the cohort was larger than the previous studies. Serum phosphate measurements were done at multiple time-points to get an exact idea of serum phosphate level. In addition, renal handling of phosphate during critical illness was measured. The concept of the study might give some research ideas to the intensivist practicing in the resource limited setting, where total parenteral nutrition with phosphate is not available.
The limitations of index study are heterogeneity of the study subjects, which included wide age range and thus, different parameters of normality of serum phosphate had to be taken. It might have been better if association between hypophosphatemia and prolonged ICU stay could be analyzed on the patients who had serum phosphate measured on all 4 d, but in this way, authors would have ended up in selecting patients who had longer ICU stay. Although the authors could explore the mechanisms related to hypophosphatemia, they could not quantify the relative contribution of each of the mechanisms. The authors did not measure glycemic and volume status; these play an important role in phosphate reabsorption. They were also not able to measure other phosphaturic hormones responsible for increased phosphate excretion.
Conclusions
Hypophosphatemia is common in critically ill children and is associated with prolonged PICU stay. One of the important mechanisms responsible for hypophosphatemia is decreased tubular reabsorption and thus, increased excretion of phosphate. Further studies need to be done for better understanding as to why there is increased renal phosphate loss in critically ill children.
Also, in future, intervention studies are required to understand the impact of normalization of phosphate in terms of morbidity.
References
Bugg NC, Jones JA. Hypophosphataemia. Pathophysiology, effects and management on the intensive care unit. Anaesthesia. 1998;53:895–902.
Lichtman MA, Miller DR, Cohen J, Waterhouse C. Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann Intern Med. 1971;74:562–8.
Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ. Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care. 2010;14:R147.
Santana e Meneses JF, Leite HP, de Carvalho WB, Lopes E Jr. Hypophosphatemia in critically ill children: prevalence and associated risk factors. Pediatr Crit Care Med. 2009;10:234–8.
Kilic O, Demirkol D, Ucsel R, Citiak A, Karabocuoglu M. Hypophosphatemia and its clinical implications in critically ill children: a retrospective study. J Crit Care. 2012;27:474–9.
de Menezes FS, Leite HP, Fernandez J, Benzecry SG, de Carvalho WB. Hypophosphatemia in critically ill children. Rev Hosp Clín. 2004;59:306–11.
Slater A, Shann F, Pearson G; Pediatric Index of Mortality (PIM) Study Group. PIM2: a revised version of the paediatric index of mortality. Intensive Care Med. 2003;29:278–85.
Walton RJ, Bijvoet OL. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;2:309–10.
Liamis G, Milionis HJ, Elisfa M. Medication-induced hypophosphatemia: a review. QJM. 2010;103:449–59.
Worley G, Claerhout SJ, Combs SP. Hypophosphatemia in malnourished children during refeeding. Clin Pediatr. 1998;37:347–52.
Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
WHO anthroplus manual. Available at: http://www.who.int/entity/growthref/tools/who_anthroplus_manual.pdf. Accessed 10 Jun 2013.
Bhutia TD, Lodha R, Kabra SK. Abnormalities in glucose homeostasis in critically ill children. Pediatr Crit Care Med. 2013;14:e16–25.
Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for haemodynamic support of paediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88.
The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8.
Aubier M, Murciano D, Lecocguic Y, et al. Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N Engl J Med. 1985;313:420–4.
Gravelyn TR, Brophy N, Siegert C, Peters-Golden M. Hypophosphatemia and phosphorous depletion in respiratory muscle weakness in general inpatient population. Am J Med. 1988;84:870–6.
Manary MJ, Hart CA, Whyte MP. Severe hypophosphatemia in children with kwashiorkor is associated with increased mortality. J Pediatr. 1998;133:789–91.
Yoshimatsu S, Hossain MI, Islam MM, et al. Hypophosphatemia among severely malnourished children with sepsis in Bangladesh. Pediatr Int. 2013;55:79–84.
Shor R, Halabe A, Rishver S, et al. Severe hypophosphatemia in sepsis as a mortality predictor. Ann Clin Lab Sci. 2006;36:67–72.
Riedler GF, Scheitlin WA. Hypophosphataemia in septicaemia: higher incidence in gram-negative than in gram-positive infections. Br Med J. 1969;1:753–6.
Antachopoulos C, Margeli A, Giannaki M, Bakoula C, Liakopoulou T, Papassotiriou I. Transient hypophosphataemia associated with acute infectious disease in paediatric patients. Scand J Infect Dis. 2002;34:836–9.
Andrade L, Rebouças NA, Seguro AC. Down-regulation of Na + transporters and AQP2 is responsible for acyclovir-induced polyuria and hypophosphatemia. Kidney Int. 2004;65:175–83.
Watanabe T, Yoshikawa H, Yamazaki S, Abe Y, Abe T. Secondary renal Fanconi syndrome caused by valproate therapy. Pediatr Nephrol. 2005;20:814–7.
Bech A, Blans M, Telting D, Boer H. Incidence and aetiology of renal phosphate loss in patients with hypophosphatemia in the intensive care unit. Intensive Care Med. 2013;39:1785–91.
Srinivasagam D, Seshadri MS, Peter JV, Cherian AM, Charles D, Kanagasabapathy AS. Prevalance & pathogenesis of hypophosphatemia in ventilated patients. Indian J Med Res. 1992;96:87–90.
Mostellar ME, Tuttle EP Jr. Effects of alkalosis on plasma concentration and urinary excretion of inorganic phosphate in man. J Clin Invest. 1964;43:138–49.
Brautbar N, Leibovici H, Massry SG. On the mechanism of hypophosphatemia during acute hyperventilation: evidence for increased muscle glycolysis. Miner Electrolyte Metab. 1983;9:45–50.
Contributions
RL, SKK and SKS participated in design of study. RL and SKS performed data interpretation, statistical analysis and drafted the manuscript. SKS collected the patient data. NG and MI were responsible for the lab work. All authors read and approved the final manuscript. RL will act as guarantor for the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
Intramural support form Department of Pediatrics, All India Institute of Medical Sciences, New Delhi.
Rights and permissions
About this article
Cite this article
Shah, S.K., Irshad, M., Gupta, N. et al. Hypophosphatemia in Critically Ill Children: Risk Factors, Outcome and Mechanism. Indian J Pediatr 83, 1379–1385 (2016). https://doi.org/10.1007/s12098-016-2188-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-016-2188-x