Abstract
Necrotizing enterocolitis (NEC), an inflammatory bowel necrosis of preterm infants, is the most common gastrointestinal emergency and a major cause of morbidity and mortality in these infants. In this article, the authors review the pathophysiology and clinical presentation of NEC and provide a critical appraisal of the evidence supporting various prophylactic and therapeutic strategies. A literature search was performed using the databases PubMed, EMBASE, and Scopus. Current pathophysiological models of NEC suggest that the disease occurs when mucosal injury in the preterm intestine results in translocation of luminal bacteria across the epithelial barrier, triggering an exaggerated and damaging local inflammatory response. Medical management of NEC is largely supportive and likely does not modify the etiopathogenesis of this disease. Antenatal steroids, human milk feedings, adoption of standardized feeding regimens, and probiotics hold promise for prevention of NEC. Future research should focus on early recognition that occurs well before the onset of intestinal necrosis, and prevention of this disease.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is an inflammatory bowel necrosis of premature infants and is a major cause of morbidity and mortality in neonatal intensive care units throughout the world [1]. In this article, the authors review the pathophysiology and clinical manifestations of NEC, and discuss the quality of evidence and strength of recommendations for the clinical management of NEC. A literature search was performed using the databases PubMed, EMBASE, and Scopus. To avoid bias in identification of existing studies, keywords were carefully short-listed prior to the actual search from anecdotal experience and from PubMed’s Medical Subject Heading (MeSH) thesaurus.
Incidence
The incidence of NEC has been estimated in population studies to be approximately 1 to 3 per 1,000 live births. In the United States, multicentric studies from the Neonatal Research Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development have reported the incidence of NEC as 7–11 % [2–4]. Similar figures (7.4 %) have been reported from the Vermont Oxford Network, which included data from 71,808 very low birth weight (VLBW) infants during a study period 2005–2006 [5]. Studies from Europe show lower incidence, ranging between 3 and 5.8 % [6, 7]. The Canadian Neonatal Network documented that 5.1 % of infants born earlier than 33 wk gestation developed NEC during 2003–2008 in a cohort of 16,669 infants [8]. The incidence of NEC varies significantly between nurseries, occurring at a stable endemic rate in each center that is punctuated by outbreaks [3].
Pathophysiology
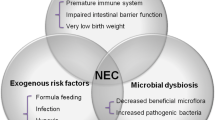
Although the etiopathogenesis of NEC remains unclear, current evidence supports a complex, multi-factorial model of disease (Fig. 1). In the following section, the authors summarize this information on factors that may predispose the developing intestine to NEC.
Prematurity
More than 90 % of all cases of NEC occur in premature infants [9]. The incidence and severity of NEC rise in inverse relationship to gestational maturity, presumably related to immaturity of gut motility, digestion, perfusion, barrier function and immune defense.
Genetic Factors
Although the rate of NEC in identical twins is higher than the general population, the influence of genetic factors is small. Bhandari et al. reported NEC in either one or both of the twins in 9 (14 %) of 63 pairs of monozygotic twins and in 29 (15 %) of 189 pairs of dizygotic twins [10]. After controlling for covariates, the genetic factors were not significant. NEC has been associated with single nucleotide polymorphisms (SNPs) in the IL-4 receptor (+1902G; protective), IL-18 (-607A; increased severity), vascular endothelial growth factor (+450C; increased risk), and carbamoyl-phosphate synthetase 1 (T450N; increased risk) [11].
Enteral Feedings
Although NEC can occur in neonates who have never been fed, 90–95 % of cases occur in infants with a history of recent volume advancement or re-initiation of enteral feedings. The introduction of feedings may cause osmotic damage to the mucosa, may alter blood flow and/or motility, and promote bacterial overgrowth in the gut lumen. Formula-fed infants are at higher risk of NEC than exclusively breast-fed infants, which has been attributed to a lack of immunoprotective factors in formula and abnormal bacterial colonization. However, despite these data, clinical trials have failed to show an association of NEC with aggressive increase in feeding volumes. Most studies indicate that early, low-volume feedings are not only safe, but may also reduce other morbidities associated with prematurity [12, 13].
Mucosal Injury
Histopathologically, NEC is characterized by coagulative necrosis, mucosal edema, ulceration, focal hemorrhages, and leukocyte infiltration. The prominence of coagulative necrosis in NEC indicates that ischemic events may play a role in NEC. However, ischemic events are recorded only in a minority of preterm infants with NEC. In contrast, NEC in full-term neonates is associated with congenital heart disease, recorded hypoxic–ischemic events, and polycythemia, factors that may plausibly result in gut hypoperfusion [14]. In growth-restricted fetuses, placental insufficiency and abnormal Doppler flow in the umbilical artery have been associated with NEC [15–19]. Dorling et al. reviewed 14 independent case series of fetuses with absence/reversal of umbilical arterial Doppler flow and showed increased odds of NEC compared with controls (odds ratio: 2.13, 95 % CI 1.49 to 3.03) [20]. The absence or reversal of diastolic blood flow in the umbilical artery is presumably associated with decreased splanchnic perfusion and ischemic intestinal injury,
Inflammatory Response
During NEC, mucosal injury results in bacterial translocation and a severe, unregulated inflammatory response [21]. Emerging evidence indicates that the activation of Toll-like receptors is an important event, which triggers the activation of the transcription factor nuclear factor-kappa B (NF-κB) [22]. Increased expression of tumor necrosis factor and platelet activating factor (PAF) propagate mucosal injury, which triggers a cascade of inflammatory mediators including IL-1β, IL-6, IL-8, IL-10, IL-12, and IL-18 [23]. Activation of the complement and coagulation cascades, cytokines, reactive oxygen species and nitric oxide further amplify the mucosal injury [23].
Bacterial Translocation
NEC always occurs after the postnatal bacterial colonization of the gut mucosa; intestinal injury in utero prior to colonization may cause strictures or atresia, but not NEC [24]. In tissue sections of NEC, bacterial overgrowth, and pneumatosis intestinalis, the accumulation of gaseous products of bacterial fermentation in the bowel wall, are readily evident. The pathogenic role of bacteria in NEC is also supported by evidence that enteral antibiotics can reduce the incidence of NEC [25].
Cases of NEC are often clustered in time and space in NICUs, which has led to suggestions that NEC may be caused by a transmissible agent. However, most studies have failed to consistently implicate a single agent. Cultures of blood and other sterile fluids from infants with NEC usually yield microorganisms that typically colonize critically-ill preterm infants and the NICU microenvironment [26]. However, some recent studies suggest that early colonization with specific Enterobacteriaceae and Clostridia may predict later development of NEC [27]. Cronobacter is another emerging Gram-negative pathogen associated with NEC [28]. Using PCR-based methods, Wang et al. demonstrated that infants who developed NEC had lower bacterial diversity and bacterial dysbiosis with abundant gammaproteobacteria (which include Enterobacteriaceae and Pseudomonadaceae) but decreased Firmicutes [29]. The role of bacterial flora in NEC remains an issue of scientific debate. The oligoclonality of gut microbiota and the disproportionate representation of coliforms may be related to broad-spectrum antibiotics, delayed or interrupted feedings, and exposure to the selected multi-drug resistant nursery flora. In a recent analysis, empirical antibiotic treatment for ≥ 4 d in the presence of sterile body fluid cultures was associated with increased risk of NEC (odds ratio 1.34, 95 % CI: 1.04–1.73) [30].
Maternal Chorioamnionitis
Several studies have shown an important association between clinical chorioamnionitis and NEC (odds ratio 1.24; 95 % CI, 1.01–1.52; p = 0.04). The association between histological chorioamnionitis with fetal involvement is also significant (OR, 3.29; 95 % CI, 1.87–5.78; p ≤ 0.0001) [31].
Red Cell Transfusions
A number of retrospective studies in the last 8 y suggest that red cell transfusions are temporally-associated with NEC in preterm infants [32]. In meta-analysis, Mohamed and Shah [33] confirmed increased risk of NEC within a 48 h period after red blood cell (RBC) transfusion (pooled odds ratio 3.91, CI: 2.97–5.14). RBC transfusions can dampen the normal postprandial increase in mesenteric blood flow in premature infants, particularly in those with a birth weight < 1,250 g [34].
Pathology
The disease is commonly localized to the ileo-colic region, although the colon may be frequently involved in term infants. In some extremely-low-birth-weight (ELBW) infants as well as in some advanced cases, there might be total gut necrosis (‘NEC totalis’). Lesions are characterized by coagulation necrosis, bacterial overgrowth, pneumatosis intestinalis, inflammation, and, depending on the age of the lesions, reparative changes.
Clinical Features
The presenting signs of NEC are protean and may be insidious in onset or sudden and catastrophic. NEC characteristically presents by 2 wk of age, but the onset may be as late as 3 mo of age in some infants [9]. Age of onset is typically inversely related to gestational age. Presenting signs include tachycardia, apnea, lethargy and temperature instability. Related gastrointestinal signs may include feeding intolerance, increased pre-feed residuals or delayed gastric emptying, emesis, abdominal distention and/or tenderness, and ileus with decreased bowel sounds. Grossly bloody stools are seen in approximately 25 % of infants. The spectrum of illness ranging from mild disease to severe NEC is commonly staged by using the modified Bell’s criteria (Table 1) [35]. Characteristically, NEC follows an initial, early stage of systemic inflammatory response, followed by a ‘definite’ stage of localized peritonitis and finally an advanced stage of diffuse peritonitis.
NEC in full-term infants: Approximately 10 % of infants with NEC are born at full-term. Unlike preterm infants, most term infants develop NEC within the first week (median 2 d) and often have colonic involvement [14]. NEC in term infants is usually ‘secondary,’ associated with birth asphyxia, polycythemia, congenital heart disease, rotavirus infections, Hirschsprung’s disease, and during withdrawl from maternal opioid narcotics [14]. Overall, the outcome is generally better than in preterm neonates.
Diagnosis
A very high index of suspicion in diagnosing at-risk infants is crucial. Most clinical antecedents prior to Bell stage III NEC are non-specific for gastrointestinal pathology and may not provide sufficient time to the clinician for early institution of treatment measures. Christensen et al. reviewed the records from 118 patients with Stage III NEC [36]. The earliest recognized antecedents were non-specific for NEC, including apnea/bradycardia, skin mottling and irritability, which were first noted 2.8 ± 2.1, 4.5 ± 3.1 and 5.4 ± 3.7 (mean ± standard deviation) h, respectively, prior to the diagnosis of NEC. The most commonly identified gastrointestinal antecedents were blood in the stools, increased abdominal girth and elevated pre-feeding gastric residuals or emesis identified 2.0 ± 1.9, 2.8 ± 3.1, and 4.9 ± 4.0 h before NEC was recognized.
Radiographic features are the mainstay of definitive diagnosis of NEC. The pathognomonic signs for NEC are pneumatosis intestinalis (Fig. 2a) and portal venous gas (Fig. 2b). Sonographic detection of portal air can also help in early diagnosis. Serial radiographs are clinically invaluable in following the progression of disease, particularly in the first 2 d after the onset of NEC [37].
Most patients with NEC develop leukocytosis and neutrophilia, although neutropenia can occur in advanced disease due to the migration of neutrophils into the peritoneal cavity. Some patients with transfusion-associated NEC may show eosinophilia. Blood cultures may grow organisms typically associated with late-onset sepsis. Thrombocytopenia may occur in stage II and III and patients with advanced NEC may have evidence of disseminated intravascular coagulation.
Prevention
The levels of evidence and strength of recommendations are summarized in Table 2. A summary of various preventive strategies is provided in Table 3.
Treatment
Medical Management
Rapid initiation of therapy is necessary for suspected as well as proven cases of NEC. There is no definitive treatment for established NEC, therefore treatment is directed at supportive care and prevention of further injury with cessation of feeding, nasogastric decompression and administration of intravenous fluids. Infants are usually made nil per os (NPO) for a variable period of time, depending on the severity of disease. Parenteral antibiotics are widely used for the treatment of NEC, but there is surprisingly sparse evidence guiding the choice of antimicrobial agent and duration of therapy. One study comparing alternative treatment regimens, that included 90 infants with definite NEC, treated 46 cases with ampicillin and gentamicin, while another 44 cases received cefotaxime and vancomycin. Infants ≥ 2,200 g birthweight had similar outcomes with either regimen. Smaller infants given cefotaxime and vancomycin had a lower risk of culture-positive peritonitis, were less likely to die or develop thrombocytopenia. These data suggest that carefully chosen antibiotic regimens can improve the outcome of NEC [38]. Antibiotic coverage for anaerobes should be considered for infants with stage III NEC.
Surgical Management
Approximately 20–40 % of patients with pneumatosis intestinalis will require surgical management. Indications for surgery include evidence of perforation seen on abdominal radiographs or positive abdominal paracentesis (stool or organism on Gram stain from peritoneal fluid). Failure of medical management, a single fixed bowel loop on radiographs, abdominal wall erythema or a palpable mass are all relative indications for surgery. In rare cases, the entire intestine can be involved, precluding surgical intervention. Ideally, surgery should be performed after the development of bowel necrosis, but before perforation and peritonitis occurs.
In unstable premature infants with perforated NEC, peritoneal drainage (PD) can be cautiously considered as an alternative to exploratory laparotomy (LAP), although the best surgical approach in these infants remains unresolved. In the NECSTEPS trial, there was no significant difference in 90 d survival, dependence on parenteral nutrition, or length of hospital stay in 117 VLBW infants randomly assigned to PD or LAP [39]. However, other studies have raised important concerns about the routine use of PD. In the NET trial, 69 ELBW patients were randomized to PD or LAP and no significant differences were noted in survival, hospital length of stay, ventilator dependence, or need for parenteral nutrition [40]. However, PD was effective as a definitive treatment in only 4/35 (11 %) surviving neonates, the rest either required a delayed laparotomy (26/34, 74 %) or died. In a recent prospective multi-center study, PD has also been associated with increased risk of death or neurodevelopmental impairment [41]. A meta-analysis of 3 prospective observational studies and 2 RCTs suggested a significant excess mortality of 55 % associated with PD [42]. There is a need for better identification of patients who are less likely to tolerate LAP and may benefit from PD as a temporizing strategy.
Prognosis
Mortality rates range between 20 and 50 %. Approximately 27–63 % of affected infants may require surgery [11, 43] and as many as 50 % infants may die in the post-operative period [43]. Subacute complications include strictures (9–36 %), recurrent NEC (5 %), dysmotility, malabsorption, short bowel syndrome, and cholestatic liver disease [44, 45]. Severe NEC has been associated with growth delay that can persist beyond infancy into childhood and poor neurodevelopmental outcome [46, 47].
Conclusions
Despite advancements in neonatal intensive care, NEC remains a devastating condition for many infants. Although the etiology of NEC remains unclear, current evidence indicates that antenatal steroids, human milk feedings, adoption of standardized feeding regimens, and probiotics hold promise for prevention of NEC. Current medical management of NEC is largely supportive and likely does not modify the etiopathogenesis of this disease. Controversies remain regarding optimal surgical management for this condition. Future research should focus on early recognition that occurs well before the onset of intestinal necrosis, and prevention of this disease.
References
Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64.
Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al; NICHD Neonatal Research Network. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147 e1-8.
Stoll BJ. Epidemiology of necrotizing enterocolitis. Clin Perinatol. 1994;21:205–18.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44:1072–5. discussion 5–6.
EXPRESS Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: Extremely preterm infants study in Sweden (EXPRESS). Acta Paediatr. 2010;99:978–92.
Bajwa NM, Berner M, Worley S, Pfister RE; Swiss Neonatal Network. Population based age stratified morbidities of premature infants in Switzerland. Swiss Med Wkly. 2011;141:w13212.
Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK; Canadian Neonatal Network. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–304.
Sharma R, Hudak ML, Tepas JJ 3rd, Wludyka PS, Marvin WJ, Bradshaw JA, et al. Impact of gestational age on the clinical presentation and surgical outcome of necrotizing enterocolitis. J Perinatol. 2006;26:342–7.
Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, et al; Neonatal Genetics Study Group. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–6.
Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–83.
Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2013;5, CD001970.
Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2013;3, CD000504.
Christensen RD, Lambert DK, Baer VL, Gordon PV. Necrotizing enterocolitis in term infants. Clin Perinatol. 2013;40:69–78.
Kirsten GF, van Zyl N, Smith M, Odendaal H. Necrotizing enterocolitis in infants born to women with severe early preeclampsia and absent end-diastolic umbilical artery doppler flow velocity waveforms. Am J Perinatol. 1999;16:309–14.
Baschat AA, Gembruch U, Reiss I, Gortner L, Weiner CP, Harman CR. Relationship between arterial and venous Doppler and perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol. 2000;16:407–13.
Soregaroli M, Bonera R, Danti L, Dinolfo D, Taddei F, Valcamonico A, et al. Prognostic role of umbilical artery Doppler velocimetry in growth-restricted fetuses. J Matern Fetal Neonatal Med. 2002;11:199–203.
Bhatt AB, Tank PD, Barmade KB, Damania KR. Abnormal Doppler flow velocimetry in the growth restricted foetus as a predictor for necrotising enterocolitis. J Postgrad Med. 2002;48:182–5. discussion 5.
Dogra S, Mukhopadhyay K, Narang A. Feed intolerance and necrotizing enterocolitis in preterm small-for-gestational age neonates with normal umbilical artery Doppler flow. J Trop Pediatr. 2012;58:513–6.
Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Arch Dis Child Fetal Neonatal Ed. 2005;90:F359–63.
Mohankumar K, Kaza N, Jagadeeswaran R, Garzon SA, Bansal A, Kurundkar AR, et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol. 2012;303:G93–102.
Frost BL, Caplan MS. Necrotizing enterocolitis: Pathophysiology, platelet-activating factor, and probiotics. Semin Pediatr Surg. 2013;22:88–93.
Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: Clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23.
Hsueh W, Caplan MS, Tan X, MacKendrick W, Gonzalez-Crussi F. Necrotizing enterocolitis of the newborn: Pathogenetic concepts in perspective. Pediatr Dev Pathol. 1998;1:2–16.
Bury RG, Tudehope D. Enteral antibiotics for preventing necrotizing enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev. 2001;1:CD000405.
Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2000;97:6043–8.
de la Cochetiere MF, Piloquet H, des Robert C, Darmaun D, Galmiche JP, Roze JC. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: The putative role of clostridium. Pediatr Res. 2004;56:366–70.
Hunter CJ, Bean JF. Cronobacter: An emerging opportunistic pathogen associated with neonatal meningitis, sepsis and necrotizing enterocolitis. J Perinatol. 2013;33:581–5.
Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–54.
Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al; NICH Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66.
Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: A systematic review and meta-analysis. J Pediatr. 2013;162:236–42. e2.
Amin SC, Remon JI, Subbarao GC, Maheshwari A. Association between red cell transfusions and necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2012;25:S85–9.
Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: A meta-analysis of observational data. Pediatrics. 2012;129:529–40.
Krimmel GA, Baker R, Yanowitz TD. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. Am J Perinatol. 2009;26:99–105.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr Clin N Am. 1986;33:179–201.
Christensen RD, Wiedmeier SE, Baer VL, Henry E, Gerday E, Lambert DK, et al. Antecedents of Bell stage III necrotizing enterocolitis. J Perinatol. 2010;30:54–7.
Frey EE, Smith W, Franken EA Jr, Wintermeyer KA. Analysis of bowel perforation in necrotizing enterocolitis. Pediatr Radiol. 1987;17:380–2.
Scheifele DW, Ginter GL, Olsen E, Fussell S, Pendray M. Comparison of two antibiotic regimens for neonatal necrotizing enterocolitis. J Antimicrob Chemother. 1987;20:421–9.
Moss RL, Dimmitt RA, Barnhart DC, Sylvester KG, Brown RL, Powell DM, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354:2225–34.
Rees CM, Eaton S, Kiely EM, Wade AM, McHugh K, Pierro A. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. 2008;248:44–51.
Blakely ML, Tyson JE, Lally KP, McDonald S, Stoll BJ, Stevenson DK, et al; NICHD Neonatal Research Network. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: Outcomes through 18 mo adjusted age. Pediatrics. 2006;117:e680–7.
Sola JE, Tepas JJ 3rd, Koniaris LG. Peritoneal drainage versus Laparotomy for Necrotizing enterocolitis and intestinal perforation: a meta-analysis. J Surg Res. 2010;161:95–100.
Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med. 2009;60:111–24.
Amin SC, Pappas C, Iyengar H, Maheshwari A. Short bowel syndrome in the NICU. Clin Perinatol. 2013;40:53–68.
Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clin Perinatol. 2013;40:135–48.
Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al; NICHD Neonatal Research Network. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703.
Pike K, Brocklehurst P, Jones D, Kenyon S, Salt A, Taylor D, et al. Outcomes at 7 y for babies who developed neonatal necrotising enterocolitis: The ORACLE Children Study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F318–22.
Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3: CD004454.
Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2013;3: CD001241.
Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: A systematic review and meta-analysis of observational studies. Arch Dis Child Fetal Neonatal Ed. 2005;90:F147–51.
Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: Systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F169–75.
Foster J, Cole M. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth-weight neonates. Cochrane Database Syst Rev. 2004:1:CD001816.
Moe-Byrne T, Wagner JV, McGuire W. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2012:3:CD001457.
Dvorak B. Epidermal growth factor and necrotizing enterocolitis. Clin Perinatol. 2004;31:183–92.
Christensen RD, Havranek T, Gerstmann DR, Calhoun DA. Enteral administration of a simulated amniotic fluid to very low birth weight neonates. J Perinatol. 2005;25:380–5.
Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011:3:CD005496.
Srinivasjois R, Rao S, Patole S. Prebiotic supplementation in preterm neonates: Updated systematic review and meta-analysis of randomised controlled trials. Clin Nutr. 2013;32:958–65.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasivajjula, H., Maheshwari, A. Pathophysiology and Current Management of Necrotizing Enterocolitis. Indian J Pediatr 81, 489–497 (2014). https://doi.org/10.1007/s12098-014-1388-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-014-1388-5