Abstract
Introduction
Hand–foot syndrome (HFS) is a limiting toxicity of capecitabine, which is not life-threatening but could compromise capecitabine efficacy.
Materials and methods
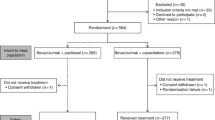
This phase II, multicenter, non-controlled study assessed the safety, particularly grade three HFS incidence, and efficacy of four capecitabine-based chemotherapy regimens [cisplatin/capecitabine (CX), epirubicin/cisplatin/capecitabine (ECX), epirubicin/oxaliplatin/capecitabine (EOX) and docetaxel/cisplatin/capecitabine (DCX)] as first-line treatment for advanced and/or metastatic gastric cancer.
Results
One hundred and eight patients were assigned to one of the four treatment groups, according to investigator’s criteria, and grouped together for both safety and efficacy primary analyses. HFS was reported in 31 patients (19.6 %) and its first presentation occurred at a median of 72 days (range 19–209 days). Grade 3 HFS developed in 6.3, 5.2, 3.7 and 2.4 %, of patients receiving ECX, DCX, EOX or CX chemotherapy regimen, respectively. Capecitabine dose reduction/discontinuation due to HFS was required in 5.7 % of patients (9/158). The most common (>10 %) grade 3–4 treatment-related AEs were neutropenia (15.2 %), asthenia (12.0 %) and diarrhoea (11.4 %).
Conclusions
A moderate incidence of HFS was reported in patients treated with capecitabine, which generally presented late and required dose reduction in <1/3 of patients. The results suggest that capecitabine may be useful in combination with standard fluorouracil-based regimens in patients with advanced and/or metastatic gastric cancer with favourable safety profile.


Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917
Cabanes A, Perez-Gomez B, Aragones N, Pollan M, Lopez-Abente G (2009) La situación del cáncer en España, 1975–2006. INE-Madrid
Altekruse SF, Kosary CL, Krapcho M et al (2011) SEER cancer statistics review, 1975–2007, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010
Hendlisz A, Bleiberg H (1995) Diagnosis and treatment of gastric cancer. Drugs 49(5):711–720
Schipper DL, Wagener DJ (1996) Chemotherapy of gastric cancer. Anticancer Drugs 7(2):137–149
Lacave AJ, Baron FJ, Anton LM et al (1991) Combination chemotherapy with cisplatin and 5-fluorouracil 5-day infusion in the therapy of advanced gastric cancer: a phase II trial. Ann Oncol 2(10):751–754
Kim NK, Park YS, Heo DS et al (1993) A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer 71(12):3813–3818
Webb A, Cunningham D, Scarffe JH et al (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15(1):261–267
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24(31):4991–4997
Hong YS, Song SY, Lee SI et al (2004) A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol 15(9):1344–1347
Koizumi W, Saigenji K, Ujiie S, Terashima M, Sakata Y, Taguchi T (2003) A pilot phase II study of capecitabine in advanced or recurrent gastric cancer. Oncology 64(3):232–236
Sakamoto J, Chin K, Kondo K et al (2006) Phase II study of a 4-week capecitabine regimen in advanced or recurrent gastric cancer. Anticancer Drugs 17(2):231–236
Kim TW, Kang YK, Ahn JH et al (2002) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol 13(12):1893–1898
Lee J, Im YH, Cho EY et al (2008) A phase II study of capecitabine and cisplatin (XP) as first-line chemotherapy in patients with advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol 62(1):77–84
Kang YK, Ryu MH, Yoo C et al (2011) Phase I/II study of a combination of docetaxel, capecitabine, and cisplatin (DXP) as first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol 67(6):1435–1443
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358(1):36–46
Kang YK, Kang WK, Shin DB et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20(4):666–673
National Institute for Health and Clinical Excellence (2010) Capecitabine for the treatment of advanced gastric cancer. NICE technology appraisal guidance 191 www.nice.org.uk/guidance/TA191. Accessed 19 Jan 2011
Webster-Gandy JD, How C, Harrold K (2007) Palmar-plantar erythrodysesthesia (PPE): a literature review with commentary on experience in a cancer centre. Eur J Oncol Nurs 11(3):238–246
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 92(3):205–216
Van Cutsem E, Twelves C, Cassidy J et al (2001) Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19(21):4097–4106
Nagore E, Insa A, Sanmartin O (2000) Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand–foot’) syndrome. Incidence, recognition and management. Am J Clin Dermatol 1(4):225–234
Abushullaih S, Saad ED, Munsell M, Hoff PM (2002) Incidence and severity of hand–foot syndrome in colorectal cancer patients treated with capecitabine: a single-institution experience. Cancer Invest 20(1):3–10
Capecitabine -SPC (2010) Xeloda (capecitabine)–Roche Pharma AG. Summary of Product Characteristics
Oxaliplatin-SPC (2010) Oxaliplatin. Summary of Product Characteristics
Epirubicin-SPC (2010) Epirubicin. Summary of Product Characteristics
Martinez Amores B, Alsina M, Jiménez E et al (2011) Observational transversal study to relate functional status and age with the doublet or triplet chemotherapy based on capecitabine in advanced gastric cancer patients. J Clin Oncol 29: (suppl 4), abstract 59
Acknowledgments
This study was supported by Roche Farma S. A. Other institutions and principal investigators who participated in the study are as follows: Alberto Abad. Oncology service. Germans Trias i Pujol Hospital. Barcelona, Spain; Alberto Arizcun. Oncology service. Palencia Hospital. Palencia, Spain; Emilio Fonseca, Oncology service. Clínico de Salamanca Hospital. Salamanca, Spain; María Gómez–Reina. Oncology service. Puerta del Mar Hospital. Cadiz, Spain; Raquel Guardeño. Oncology service. ICO Gerona (Josep Trueta) Hospital. Gerona, Spain; Purificación Martínez. Basurto Hospital. Bilbao, Spain; Miguel Méndez, Oncology service. Móstoles Hospital. Madrid, Spain; Jesús García-Foncillas López. Oncology service. Clínical University of Navarra (CUN). Navarra, Spain; Luis López-Gómez. Oncology service. Complejo Virgen de la Salud Hospital. Toledo, Spain; Jose L. García López. Oncology service. Ramón y Cajal Hospital. Madrid, Spain; Rodrigo Lastra del Prado. Oncology service. San Jorge Hospital. Huesca, Spain; Amparo Oltra. Oncology service. Virgen de los Lirios Hospital. Alicante, Spain; Pedro Salinas Hernández. Oncology service. Grupo Hospitalario Quirón. Madrid, Spain; Mª Antonieta Salud. Oncology service. Universitari Arnau de Vilanova Hospital. Lleida, Spain; Marina Alonso. Oncology service. San Pedro Hospital. Logroño, Spain.; Isabel Busquier. Oncology service. Provincial de Castellón Hospital,. Castellón, Spain; Nuria Cárdenas. Oncology service. San Cecilio Hospital. Granada, Spain; Javier Castellanos. “Xeral Cíes” University Hospital. Vigo, Spain; Teresa García García. Oncology service. Morales Meseguer H. Murcia, Spain; Alicia Hurtado. Oncology service. Fundación Alcorcón Hospital. Madrid, Spain; Antonio Lorenzo Peñuelas. Oncology service. Puerto Real Hospital. Cadiz, Spain; Jose Ramón Mel Lorenzo. Oncology service. Xeral Calde de Lugo Hospital. Lugo, Spain; Manuel Ramos Vazquez. Oncology service. Oncologic Centre of de Galicia “José Antonio Quiroga y Piñeyro”. La Coruña, Spain; Ruth Vera. Oncology service. Navarra Hospital. Pamplona, Spain.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Martin, C., Sánchez, A., Irigoyen, A. et al. Incidence of hand–foot syndrome with capecitabine in combination with chemotherapy as first-line treatment in patients with advanced and/or metastatic gastric cancer suitable for treatment with a fluoropyrimidine-based regimen. Clin Transl Oncol 14, 689–697 (2012). https://doi.org/10.1007/s12094-012-0858-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0858-3