Abstract
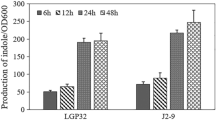
Cell to cell communication facilitated by chemical signals plays crucial roles in regulating various cellular functions in bacteria. Indole, one such signaling molecule has been demonstrated to control various bacterial phenotypes such as biofilm formation and virulence in diverse bacteria including Vibrio cholerae. The present study explores some key factors involved in indole production and the subsequent pathogenesis of V. cholerae. Indole production was higher at 37 °C than at 30 °C, although the growth at 37 °C was slightly higher. A positive correlation was observed between indole production and biofilm formation in V. cholerae. Maximum indole production was detected at pH 7. There was no significant difference in indole production between clinical and environmental V. cholerae isolates, although indole production in one environmental isolate was significantly different. Both growth and indole production showed relevant changes with differences in salinity. An indole negative mutant strain was constructed using transposon mutagenesis and the direct effect of indole on the virulence of V. cholerae was evaluated using Galleria mellonella larvae model. Comparison to the wild type strain, the mutant significantly reduced the mortality of G. mellonella larvae which regained its virulence after complementation with exogenous indole. A gene involved in indole production and the virulence of V. cholerae was identified.




Similar content being viewed by others
References
Camilli A, Bassler BL (2006) Bacterial small-molecule signaling pathways. Science 311:1113–1116. doi:10.1126/science.1121357
Lee JH, Lee J (2010) Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. doi:10.1111/j.1574-6976.2009.00204.x
Mueller RS, Beyhan S, Saini SG, Yildiz FH, Bartlett DH (2009) Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae. J Bacteriol 191:3504–3516. doi:10.1128/JB.01240-08
Kaper JB, Morris JG, Levine MM (1995) Cholera. Clin Microbiol Rev 8:48–86
Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL (2007) The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 450:883–886. doi:10.1038/nature06284
Gong F, Yanofsky C (2002) Analysis of tryptophanase operon expression in vitro: accumulation of TnaC-peptidyl-tRNA in a release factor 2-depleted S-30 extract prevents Rho factor action, simulating induction. J Biol Chem 277:17095–17100. doi:10.1074/jbc.M201213200
Lee J, Jayaraman A, Wood TK (2007) Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 7:42. doi:10.1186/1471-2180-7-42
Yanofsky C, Horn V, Gollnick P (1991) Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J Bacteriol 173:6009–6017
Botsford JL, DeMoss RD (1971) Catabolite repression of tryptophanase in Escherichia coli. J Bacteriol 105:303–312
Kobayashi A, Hirakawa H, Hirata T, Nishino K, Yamaguchi A (2006) Growth phase-dependent expression of drug exporters in Escherichia coli and its contribution to drug tolerance. J Bacteriol 188:5693–5703. doi:10.1128/JB.00217-06
Anyanful A, Dolan-Livengood JM, Lewis T, Sheth S, Dezalia MN, Sherman MA, Kalman LV, Benian GM, Kalman D (2005) Paralysis and killing of Caenorhabditis elegans by enteropathogenic Escherichia coli requires the bacterial tryptophanase gene. Mol Microbiol 57:988–1007. doi:10.1111/j.1365-2958.2005.04739.x
Chant EL, Summers DK (2007) Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol Microbiol 63:35–43. doi:10.1111/j.1365-2958.2006.05481.x
Hirakawa H, Inazumi Y, Masaki T, Hirata T, Yamaguchi A (2005) Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol Microbiol 55:1113–1126. doi:10.1111/j.1365-2958.2004.04449.x
Han TH, Lee JH, Cho MH, Wood TK, Lee J (2011) Environmental factors affecting indole production in Escherichia coli. Res Microbiol 162:108–116. doi:10.1016/j.resmic.2010.11.005
Kawamura-Sato K, Shibayama K, Horii T, Iimuma Y, Arakawa Y, Ohta M (1999) Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol Lett 179:345–352. doi:10.1111/j.1574-6968.1999.tb08748.x
Nesper J, Lauriano CM, Klose KE, Kapfhammer D, Kraiss A, Reidl J (2001) Characterization of Vibrio cholerae O1 El tor galU and galE mutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect Immun 69:435–445. doi:10.1128/IAI.69.1.435-445.2001
Lyell NL, Dunn AK, Bose JL, Vescovi SL, Stabb EV (2008) Effective mutagenesis of Vibrio fischeri by using hyperactive mini-Tn5 derivatives. Appl Environ Microbiol 74:7059–7063. doi:10.1128/AEM.01330-08
Durai S, Pandian SK, Balamurugan K (2011) Establishment of a Caenorhabditis elegans infection model for Vibrio alginolyticus. J Basic Microbiol 51:243–252. doi:10.1002/jobm.201000303
Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC Jr, Mylonakis E (2009) Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 53:2605–2609. doi:10.1128/AAC.01533-08
Senior NJ, Bagnall MC, Champion OL, Reynolds SE, La Ragione RM, Woodward MJ, Salguero FJ, Titball RW (2011) Galleria mellonella as an infection model for Campylobacter jejuni virulence. J Med Microbiol 60:661–669. doi:10.1099/jmm.0.026658-0
Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR (1983) Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45:275–283
Alam M, Sultana M, Nair GB, Siddique AK, Hasan NA, Sack RB, Sack DA, Ahmed KU, Sadique A, Watanabe H, Grim CJ, Huq A, Colwell RR (2007) Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc Natl Acad Sci USA 104:17801–17806. doi:10.1073/pnas.0705599104
Colwell RR, Huq A (1994) Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann N Y Acad Sci 740:44–54. doi:10.1111/j.1749-6632.1994.tb19852.x
Muic V, Ljubicic M, Vodopija I, Mayer V (1999) Basing a selective method for isolating environmental Vibrio cholerae on differences in the growth rate of competing Vibrio metschnikovii. Veterinarski Arhiv 69:125–134
Li Y, Cole K, Altman S (2003) The effect of a single, temperature-sensitive mutation on global gene expression in Escherichia coli. RNA 9:518–532. doi:10.1261/rna.2198203
Cockburn TA, Cassanos JG (1960) Epidemiology of endemic cholera. Public Health Rep 75:791–803
Huq A, West PA, Small EB, Huq MI, Colwell RR (1984) Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol 48:420–424
Blankenhorn D, Phillips J, Slonczewski JL (1999) Acid- and base-induced proteins during aerobic and anaerobic growth of Escherichia coli revealed by two-dimensional gel electrophoresis. J Bacteriol 181:2209–2216
Yohannes E, Barnhart DM, Slonczewski JL (2004) pH-dependent catabolic protein expression during anaerobic growth of Escherichia coli K-12. J Bacteriol 186:192–199. doi:10.1128/JB.186.1.192-199.2004
Lyon WJ (2001) TaqMan PCR for detection of Vibrio cholerae O1, O139, non-O1, and non-O139 in pure cultures, raw oysters, and synthetic seawater. Appl Environ Microbiol 67:4685–4693. doi:10.1128/AEM.67.10.4685-4693.2001
Lee J, Attila C, Cirillo SL, Cirillo JD, Wood TK (2009) Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb Biotechnol 2:75–90. doi:10.1111/j.1751-7915.2008.00061.x
Molina-Santiago C, Daddaoua A, Fillet S, Duque E, Ramos JL (2014) Interspecies signalling: Pseudomonas putida efflux pump TtgGHI is activated by indole to increase antibiotic resistance. Environ Microbiol 16:1267–1281. doi:10.1111/1462-2920.12368
Nikaido E, Giraud E, Baucheron S, Yamasaki S, Wiedemann A, Okamoto K, Takagi T, Yamaguchi A, Cloeckaert A, Nishino K (2012) Effects of indole on drug resistance and virulence of Salmonella enterica serovar Typhimurium revealed by genome-wide analyses. Gut Pathog 4:5. doi:10.1186/1757-4749-4-5
Martin K, Morlin G, Smith A, Nordyke A, Eisenstark A, Golomb M (1998) The tryptophanase gene cluster of Haemophilus influenzae type b: evidence for horizontal gene transfer. J Bacteriol 180:107–118
Hirakawa H, Kodama T, Takumi-Kobayashi A, Honda T, Yamaguchi A (2009) Secreted indole serves as a signal for expression of type III secretion system translocators in enterohaemorrhagic Escherichia coli O157:H7. Microbiology 155:541–550. doi:10.1099/mic.0.020420-0
Dimroth P (1997) Primary sodium ion translocating enzymes. Biochim Biophys Acta 1318:11–51. doi:10.1016/S0005-2728(96)00127-2
Balsera M, Buey RM, Li XD (2011) Quaternary structure of the oxaloacetate decarboxylase membrane complex and mechanistic relationships to pyruvate carboxylases. J Biol Chem 286:9457–9467. doi:10.1074/jbc.M110.197442
Hase CC, Mekalanos JJ (1999) Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA 96:3183–3187. doi:10.1073/pnas.96.6.3183
Minato Y, Fassio SR, Wolfe AJ, Hase CC (2013) Central metabolism controls transcription of a virulence gene regulator in Vibrio cholerae. Microbiology 159:792–802. doi:10.1099/mic.0.064865-0
Snell EE (1975) Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol 42:287–333. doi:10.1002/9780470122877.ch6
Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31:224–245. doi:10.1016/j.biotechadv.2012.10.00
Koul S, Prakash J, Mishra A, Kalia VC (2016) Potential emergence of multi-quorum sensing inhibitor resistant (MQSIR) bacteria. Indian J Microbiol 56:1–18. doi:10.1007/s12088-015-0558-0
Kalia VC, Purohit HJ (2011) Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol 37:121–140. doi:10.3109/1040841X.2010.532479
Acknowledgments
This work was supported by funds from The Thailand Research Fund and Royal Golden Jubilee Ph.D. program (Grant No. PHD/0213/2556). Thanks to Dr. Brian Hodgson for assistance with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nuidate, T., Tansila, N., Saengkerdsub, S. et al. Role of Indole Production on Virulence of Vibrio cholerae Using Galleria mellonella Larvae Model. Indian J Microbiol 56, 368–374 (2016). https://doi.org/10.1007/s12088-016-0592-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-016-0592-6