Abstract
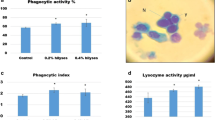
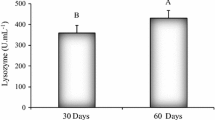
The study attempted to confirm the efficacy of Saccharomyces cerevisiae as a probiotic in augmenting the overall wellbeing and disease resistance in the Indian Major Carp, rohu (Labeo rohita) juveniles. The growth rate, nutritional quality and immunity of L. rohita fry were studied for 60 days fed with four isocaloric and isonitrogenous diets supplemented with 0.50 % (L1), 0.75 % (L2) and 1.00 % (L3) lyophilized whole yeast (S. cerevisiae) cells. L3-fed fish registered significantly better in wellbeing parameters such as growth, RNA:DNA, lower feed conversion and higher protein efficiency ratios. High intestinal enzyme (protease and α-amylase) activities, high liver serum GOT and GPT activities, and better non-specific immune responses were also demonstrated by them. The blood parameters hemoglobin, total erythrocyte and leukocyte counts, corpuscular volume, corpuscular hemoglobin, and cell hemoglobin concentration were also encouraging. Challenged with Aeromonas hydrophila AH2 (hourly exposure to 105 and 107 CFUs/ml strengths with a week interval) by bath exposure, highest survival percentage (96.66 %) was observed in L3-fed fish, whereas only 30 % in the control, after 10 days.
Similar content being viewed by others
Notes
All the values are provided on per liter basis; the final pH was adjusted to 5.5.
References
Zhang X, Shu M, Wang Y, Fu L, Li W, Deng B, Liang Q, Shen W (2014) Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J Microbiol Biotechnol 30:2523–2531. doi:10.1007/s11274-014-1677-1
Mishra S, Pattnaik P, Mohanty S, Ayyappan S (2001) Probiotics: possible applications in aquaculture. Fish Chimes 21:31–37
Navarrete P, Tovar-Ramírez D (2014) Use of yeasts as probiotics in fish aquaculture. In: Hernandez-Vergara MP, Perez-Rostro CI (ed) Sustainable aquaculture techniques. INTECH Open Science, pp 135–172. doi:10.5772/57196
Takada Y, Nishino Y, Ito C, Watanabe H, Kanzaki K, Tachibana T, Azuma M (2014) Isolation and characterization of baker’s yeast capable of strongly activating a macrophage. FEMS Yeast Res 14:261–269. doi:10.1111/1567-1364.12098
Perez-Sanchez T, Ruiz-Zarzuela I, de Blas I, Balcazar JL (2013) Probiotics in aquaculture: a current assessment. Rev Aquac. doi:10.1111/raq.1203
Rozita K, Shila S, Mahdy C (2013) Effect whole and cell wall of Saccharomyces cerevisiae in immunity factors on rainbow trout (Oncorhynchus mykiss). American-Eurasian J Agric Environ Sci 13:633–638. doi:10.5829/idosi.aejaes.2013.13.05.224
Ortuno J, Cuesta A, Rodriguez A, Esteban MA, Meseguer J (2002) Oral administration of yeast Saccharomyces cerevisiae enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Vet Immunol Immunopathol 85:41–50. doi:10.1016/S0165-2427(01)00406-8
Mari S, Samy L, Jagruthi C, Anbazahan SM, Yogeshwari G, Thirumurugan R, Arockiaraj J, Mariappan P, Balasundaram C, Harikrishnan R (2014) Protective effect of chitin and chitosan enriched diets on immunity and disease resistance in Cirrhina mrigala against Aphanomyces invadans. Fish Shellfish Immunol 39:378–385. doi:10.1016/j.fsi.2014.05.027
Pooramini M, Kamali A, Hajimoradloo A, Alizadeh M, Ghorbani R, Hatami R, Haghparast S (2014) The effects of different concentrations of probiotic Saccharomyces cerevisiae on growth performance and survival rate of rainbow trout (Oncorhynchus mykiss), fry and resistance against salinity. Afr J Biotechnol 13:1160–1168. doi:10.5897/AJB2013.12214
Josue SP, Knauth P, Zaira L, Orfil GR, Ester MRM, Victor GA, Monique L, Rosa AUB (2014) Differences in the amount of β-glucan and mannan in strains of Saccharomyces cerevisiae and Meyerozyma guilliermondii isolated from agave must used in tequila production. Microbiol Res Int 2:1–8, ISSN: 2354-2128
Sych G, Frost P, Irnazarow I (2013) Influence of β-glucan (Macrogard®) on innate immunity of carp fry. Bull Vet Inst Pulawy 57:219–223. doi:10.2478/bvip-2013-0039
De BC, Meena DK, Behera BK, Das P, Mohapatra PD, Sharma AP (2014) Probiotics in fish and shellfish culture: immunomodulatory and ecophysiological responses. Fish Physiol Biochem 40:921–971. doi:10.1007/s10695-013-9897-0
El-Zaeem SY, Amer TN, El-Tawil NE (2014) Evaluation of the productive performance characteristics of red tilapia (Oreochromis sp.) injected with shark DNA into skeletal muscles and maintained diets containing different levels of probiotic and amino yeast. Afr J Biotechnol 11:7286–7293. doi:10.1016/j.ijmm.2009.08.005
APHA (2005) Standard methods for the examination of water and wastewater. 21st Ed. Am Public Health Assoc, Washington DC, www.standardmethods.org, ISBN:0875530478
Nandeesha MC, Sentilkumar V, Antony Jesu Prabhu P (2013) Feed management of major carps in India, with special reference to practices adopted in Tamil Nadu. In: Hasan MR, New MB (eds) On-farm feeding and feed management in aquaculture. FAO Fisheries and Aquaculture Technical Paper No. 583, Rome, FAO, E-ISBN:978-92-5-107979-9, pp 433–462
AOAC (2000) Official methods of analysis. 17th Ed. Association of Official Analytical Chemists, Gaithersburg, Maryland, USA, ISBN:0935584676/9780935584677
Lavanya S, Ramesh M, Kavitha C, Malarvizhi A (2011) Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere 82:977–985. doi:10.1016/j.chemosphere.2010.10.071
Oelschlaeger TA (2010) Mechanisms of probiotic actions: a review. Int J Med Microbiol 300:57–62. doi:10.1016/j.ijmm.2009.08.005
Wang Y, Li J, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquaculture 281:1–4. doi:10.1016/j.aquaculture.2008.06.002
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14. doi:10.1016/j.aquaculture.2007.11.019
Sahu MK, Swarnakumar NS, Sivakumar K, Thangaradjou T, Kannan L (2008) Probiotics in aquaculture: importance and future perspectives. Indian J Microbiol 48:299–308. doi:10.1007/s12088-008-0024-3
Qi Z, Zhang XH, Boon N, Bossier P (2009) Probiotics in aquaculture of China—current state, problems and prospect. Aquaculture 290:15–21. doi:10.1016/j.aquaculture.2009.02.012
Balcazar JL, Vendrell D, Blas I, Ruiz-Zarzuela I, Girones O, Muzquiz JL (2007) In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet Microbiol 122:373–380. doi:10.1016/j.vetmic.2007.01.023
Denev S, Staykov Y, Moutafchieva R, Beev G (2009) Microbial ecology of the gastrointestinal tract of fish and the potential application of probiotics and prebiotics in finfish aquaculture, 1:1–29 Int Aquat Res, ISSN: 2008-4935
Aly SM, Abd-El-Rahman AM, John G, Mohamed MF (2008) Characterization of some bacteria isolated from Oreochromis niloticus and their potential use as probiotics. Aquaculture 277:1–6. doi:10.1016/j.aquaculture.2008.02.021
Zhou X, Tian Z, Wang Y, Li W (2010) Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol Biochem 36:501–509. doi:10.1007/s10695-009-9320-z
Gomez GD, Balcazar JL (2008) A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol 52:145–154. doi:10.1111/j.1574-695X.2007.00343.x
Harikrishnan R, Balasundaramb C, Heo MS (2010) Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol 29:868–874. doi:10.1016/j.fsi.2010.07.031
Kim DH, Austin B (2006) Innate immune responses in rainbow trout (Oncorhynchus mykiss, Walbaum) induced by probiotics. Fish Shellfish Immunol 21:513–524. doi:10.1016/j.fsi.2006.02.007
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14. doi:10.1016/j.fsi.2010.02.017
Wang Y (2011) Use of probiotics Bacillus coagulans, Rhodopseudomonas palustris and Lactobacillus acidophilus as growth promoters in grass carp (Ctenopharyngodon idella) fingerlings. Aquac Nutr 17:372–378. doi:10.1111/j.1365-2095.2010.00771.x
Zhou X, Wang Y, Li W (2009) Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 287:349–353. doi:10.1016/j.aquaculture.2008.10.046
Wang Y, Gu Q (2010) Effect of probiotics on white shrimp (Penaeus vannamei) growth performance and immune response. Mar Biol Res 6:327–332. doi:10.1080/17451000903300893
Tovar-Ramírez D, Mazurais D, Gatesoupe JF, Quazuguel P, Cahu CL, Zambonino-Infante JL (2010) Dietary probiotic live yeast modulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae. Aquaculture 300:142–147. doi:10.1016/j.aquaculture.2009.12.015
Zhou X, Wang Y (2012) Probiotics in aquaculture–benefits to the health, technological applications and safety. In: Carvalho ED, David GS, Silva RJ (eds) Health and environment in aquaculture. InTech, ISBN: 978-953-51-0497-1, pp 1–13. doi: 10.5772/29037
Wang Y, Xu Z (2006) Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim Feed Sci Technol 127:283–292. doi:10.1016/j.anifeedsci.2005.09.003
Acknowledgments
In terms of materials and facilities, the support and cooperation received from the CIFA, Bhubaneswar for the various wet-lab analyses, the IMMT, Bhubaneswar for the scanning electron microscopy facility, and the IMTECH, Chandigarh for the pathogenic strain used in the study are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Partha Bandyopadhyay and Snehasish Mishra: Co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bandyopadhyay, P., Mishra, S., Sarkar, B. et al. Dietary Saccharomyces cerevisiae Boosts Growth and Immunity of IMC Labeo rohita (Ham.) Juveniles. Indian J Microbiol 55, 81–87 (2015). https://doi.org/10.1007/s12088-014-0500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-014-0500-x