Abstract
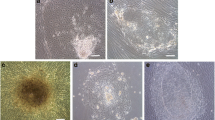
Human induced pluripotent stem cells (hiPSCs) are a type of pluripotent stem cells artificially derived from an adult somatic cell (typically human fibroblast) by forced expression of specific genes. In recent years, different feeders like inactivated mouse embryonic fibroblasts (MEFs), human dermal fibroblasts (HDFs), and feeder free system have commonly been used for supporting the culture of stem cells in undifferentiated state. In the present work, the culture of hiPSCs and their characterizations on BD Matrigel (feeder-and serum-free system), MEF and HDF feeders using cell culture methods and molecular techniques were evaluated and compared. The isolated HDFs from foreskin samples were reprogrammed to hiPSCs using gene delivery system. Then, the pluripotency ability of hiPSCs cultured on each layer was determined by teratoma formation and immunohistochemical staining. After EBs generation the expression level of three germ layers genes were evaluated by Q-real-time PCR. Also, the cytogenetic stability of hiPSCs cultured on each condition was analyzed by karyotyping and comet assay. Then, the presence of pluripotency antigens were confirmed by Immunocytochemistry (ICC) test and alkaline phosphatase staining. This study were showed culturing of hiPSCs on BD Matrigel, MEF and HDF feeders had normal morphology and could maintain in undifferentiated state for prolonged expansion. The hiPSCs cultured in each system had normal karyotype without any chromosomal abnormalities and the DNA lesions were not observed by comet assay. Moreover, up-regulation in three germ layers genes in cultured hiPSCs on each layer (same to ESCs) compare to normal HDFs were observed (p < 0.05). The findings of the present work were showed in stem cells culturing especially hiPSCs both MEF and HDF feeders as well as feeder free system like Matrigel are proper despite benefits and disadvantages. Although, MEFs is suitable for supporting of stem cell culturing but it can animal pathogens transferring and inducing immune response. Furthermore, HDFs have homologous source with hiPSCs and can be used as feeder instead of MEF but in therapeutic approaches the cells contamination is a problem. So, this study were suggested feeder free culturing of hiPSCs on Matrigel in supplemented media (without using MEF conditioned medium) resolves these problems and could prepare easy applications of hiPSCs in therapeutic approaches of regenerative medicine such as stem-cell therapy and somatic cell nuclear in further researches.









Similar content being viewed by others
Abbreviations
- HDFs:
-
Human dermal fibroblasts
- hiPSC:
-
Human induced pluripotent stem cell
- hESCs:
-
Human embryonic stem cells
- ICC:
-
Immunocytochemistry
- IHC:
-
Immunohistochemical
References
Aguilar-Gallardo C, Poo M, Gomez E, Galan A, Sanchez E, Marques-Mari A, Ruiz V, Medrano J, Riboldi M, Valbuena D, Simon C (2010) Derivation, characterization, differentiation, and registration of seven human embryonic stem cell lines (VAL-3, −4, −5, −6 M, −7, −8, and −9) on human feeder. In Vitro Cell Dev Biol Anim 46(3–4):317–326
Amit M, Margulets V, Segev H, Shariki C, Laevsky I, Coleman R, Itskovitz-Eldor J (2003) Human feeder layers for human embryonic stem cells. Biol Reprod 68:2150–2156
Bayart E, Cohen-Haguenauer O (2013) Technological overview of iPS induction from human adult somatic cells. Curr Gene Ther 13(2):73–92
Bisson F, Rochefort É, Lavoie A, Larouche D, Zaniolo K, Simard-Bisson C, Damour O, Auger FA, Guérin SL, Germain L (2013) Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int J Mol Sci 14(3):4684–4704
Chen KG, Mallon BS, McKay RDG, Robey PG (2014) Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 14(1):13–26
Cheng L, Hammond H, Ye Z, Zhan X, Dravid G (2003) Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells 21:131–142
Choi NY, Park YS, Ryu JS, Lee HJ, Araúzo-Bravo MJ, Ko K, Han DW, Schöler HR, Ko K (2014) A novel feeder-free culture system for expansion of mouse spermatogonial stem cells. Mol Cell 37(6):473–479
Crook JM, Peura TT, Kravets L, Bosman AG, Buzzard JJ, Horne R, Hentze H, Dunn NR, Zweigerdt R, Chua F, Upshall A, Colman A (2007) The generation of six clinical-grade human embryonic stem cell lines. Cell Stem Cell 1:490–494
Denham M, Leung J, Tay C, Wong RCB, Donovan P, Dottori M, Pébay A (2009) A new feeder-free technique to expand human embryonic stem cells and induced pluripotent stem cells. Open Stem Cell J 1:76–82
Ellerström C, Strehl R, Moya K, Andersson K, Bergh C, Lundin K, Hyllner J, Semb H (2006) Derivation of a xeno-free human embryonic stem cell line. Stem Cells 24:2170–2176
Ghasemi-Dehkordi P, Allahbakhshian-Farsani M, Abdian N, Mirzaeian A, Hashemzadeh-Chaleshtori M, Jafari-Ghahfarokhi H (2014) Effects of feeder layers, culture media, conditional media, growth factors, and passages number on stem cell optimization. Proc Natl Acad Sci India Sect B Biol Sci. doi:10.1007/s40011-014-0430-8
Hovatta O, Mikkola M, Gertow K, Strömberg A, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andäng M, Ahrlund‐Richter L (2003) A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod 18(7):1404–1409
Jung YW, Hysolli E, Kim KY, Tanaka Y, Park IH (2012) Human induced pluripotent stem cells and neurodegenerative disease: prospects for novel therapies. Curr Opin Neurol 25(2):125–130
King RW, Peters J-M, Tugendreich S, Rolfe M, Hieter P, Kirschner MW (1995) A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81:279–288
Kipreos ET, Lander LE, Wing JP, He WW, Hedgecock EM (1996) cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell 85:829–839
Kleinman HK, Martin GR (2005) Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15(5):378–386
McKelvey-Martin VJ, Ho ETS, McKeown SR, Johnston SR, McCarthy PJ, Rajab NF, Downes CS (1998) Emerging applications of the single cell gel electrophoresis (Comet) assay. I. Management of invasive transitional cell human bladder carcinoma. II. Fluorescent in situ hybridization Comets for the identification of damaged and repaired DNA sequences in individual cells. Mutagen 13:1–8
Pakzad M, Totonchi M, Taei A, Seifinejad A, Hassani SN, Baharvand H (2010) Presence of a ROCK inhibitor in extracellular matrix supports more undifferentiated growth of feeder-free human embryonic and induced pluripotent stem cells upon passaging. Stem Cell Rev Rep 6(1):96–107
Pan C, Hicks A, Guan X, Chen H, Bishop CE (2010) SNL fibroblast feeder layers support derivation and maintenance of human induced pluripotent stem cells. J Genet Genomics 37(4):241–248
Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451(7175):141–146
Richards M, Fong CY, Chan WK, Wong PC, Bongso A (2002) Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol 20:933–936
Robinton DA, Daley GQ (2012) The promise of induced pluripotent stem cells in research and therapy. Nature 481(7381):295–305
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 148–190
Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT (2009) Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. PNAS 106(37):15720–15725
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fi broblast cultures by defi ned factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fi broblasts by defined factors. Cell 131:861–872
Takahashi K, Narita M, Yokura M, Ichisaka T, Yamanaka S (2009) Human induced pluripotent stem cells on autologous feeders. PLoS ONE 4(12):e8067
Tecirlioglu RT, Nguyen L, Koh K, Trounson AO, Michalska AE (2010) Derivation and maintenance of human embryonic stem cell line on human adult skin fi broblast feeder cells in serum replacement medium. In Vitro Cell Dev Biol Anim 46(3–4):231–235
Unger C, Gao S, Cohen M, Jaconi M, Bergstrom R, Holm F, Galan A, Sanchez E, Irion O, Dubuisson JB (2009) Immortalized human skin fibroblast feeder cells support growth and maintenance of both human embryonic and induced pluripotent stem cells. Hum Reprod 24:2567–2581
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK (2001) Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 19:971–974
Acknowledgments
We would like to thank Rudolf Jaenisch laboratory and Tronolab for providing the plasmids. This study was supported by research Grants: 90-12-12, 91-10-5, 91-11-4, 91-11-6, and 91-12-13 of Shahrekord University of Medical Sciences, Shahrekord, Iran. The study team would like to gratefully acknowledge to the staffs of Cellular and Molecular Research Center for their sincere cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi-Dehkordi, P., Allahbakhshian-Farsani, M., Abdian, N. et al. Comparison between the cultures of human induced pluripotent stem cells (hiPSCs) on feeder-and serum-free system (Matrigel matrix), MEF and HDF feeder cell lines. J. Cell Commun. Signal. 9, 233–246 (2015). https://doi.org/10.1007/s12079-015-0289-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-015-0289-3