Abstract
Molecular recognition and aggregation occurring in solution are critical events towards the nucleation and growth of a crystal. However, controlling aggregation towards a particular supramolecular assembly is difficult due to lack of information on its thermodynamics and kinetics. Hence, the occurrence of supramolecular isomers is hardly recognized. In this paper, therefore, we demonstrate a retrosynthetic analysis to interpret the occurrence of isostructures and supramolecular isomers and predict the possibility of new phases in copper halide-pyridazine-H 2O system. A significant feature of this paper is the use of crystal engineering tools, namely, synthons and tectons to interpret the phase diagram of a system. The structure-synthesis correlation discussed here provides chemical insight to evolve a synthetic protocol to interpret and predict the possibility of supramolecular isomers in metal organic solids.

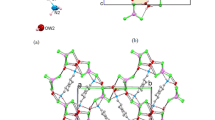
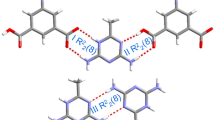
[CuII(pdz)X2], 1–2; [Cu2 I(pdz)X2], 3-5; [CuI(pdz)X], 6–8 and [Cu2 I(pdz)3Cl2].3H2O, 9 where pdz = pyridazine, were crystallized from aqueous solution for the first time and their structure elucidation was carried out using X-ray crystallographic techniques. Growth of isostructures and supramolecular isomers in 1–9 has been interpreted in terms of tectons (I–III).




Similar content being viewed by others
References
Bernstein J 2002 In Polymorphism in Molecular Crystals (New York: Oxford University Press)
Desiraju G R 1989 In Crystal engineering: The design of organic solids (Amsterdam: Elsevier)
(a) Childs S L and Hagen K S 2002 CrystEngComm 4 265; (b) Diskin-Posner Y, Patra G K and Goldberg I 2002 CrystEngComm 4 296
Black J F B, Davey R J, Gowers R J and Yeoh A 2015 CrystEngComm 17 5139
(a) Suresh K and Nangia A 2014 Cryst. Growth Des. 14 2945; (b) Swapna B, Maddileti D and Nangia A 2014 Cryst. Growth Des. 14 5991
(a) Sarkar S, Pavan M S, Cherukuvada S and Guru Row T N 2016 Chem. Commun. 52 5820; (b) Bolla G and Nangia A 2016 Chem. Commun. 52 8342; (c) Babu N J, Cherukuvada S, Thakuria R and Nangia A 2010 Cryst. Growth Des. 10 1979
Chen P K, Che Y X, Xue L and Zheng J M 2006 J. Solid State Chem. 179 2656
Zheng B, Bai J and Zhang Z 2010 CrystEngComm 12 49
Moulton B and Zaworotko M J 2001 Chem. Rev. 101 1629
Desiraju G R, Vittal J J and Ramanan A 2011 In Crystal Engineering: A Textbook (Singapore: IISc Press and World Scientific Publishing)
Singh M, Thomas J and Ramanan A 2010 Aust. J. Chem. 63 565
Singh M, Kumar D, Thomas J and Ramanan A 2010 J. Chem. Sci. 122 757
(a) Thomas J 2009 Structure-synthesis correlation of phosphomolybdates based solids: Linking molecular units and their solid-state counterparts PhD Thesis (New Delhi: Indian Institute of Technology); (b) Singh M 2011 Crystal Engineering of Metal Complex or Coordination Polymer Templated Polyoxomolybdates: Chemical Insights PhD Thesis (New Delhi: Indian Institute of Technology)
(a) Thomas J and Ramanan A 2008 Cryst. Growth Des. 8 3390; (b) Upreti S and Ramanan A 2007 Cryst. Growth Des. 7 966
Etter M C 1990 Acc. Chem. Res. 23 120
Desiraju G R 1995 Angew. Chem. Int. Ed. 34 2311
Davey R J, Allen K, Blagden N, Cross W I, Lieberman H F, Qualye M J, Righini S, Seton L and Tiddy G J T 2002 CrystEngComm 4 257
Ramanan A and Whittingham M S 2006 Cryst. Growth Des. 6 2419
(a) Pavani K, Ramanan A and Whittingham M S 2006 J. Mol. Struct. 796 179; (b) Thomas J, Kumar D and Ramanan A 2013 Inorg. Chim. Acta 396 126
(a) Xing K, Fan R, Gao S, Wang X, Du X, Wang P, Fang R and Yang Y 2016 Dalton Trans. 45 4863; (b) Zhang S, Li H, Duan E, Han Z, Li L, Tang J, Shi W and Cheng P 2016 Inorg. Chem. 55 1202; (c) Lu J Y 2003 Coord. Chem. Rev. 246 345; (d) Hoskins B F and Robson R 1990 J. Am. Chem. Soc. 112 1546
Biradha K, Ramanan A and Vittal J J 2009 Cryst. Growth Des. 9 2969
Kromp T and Sheldrick W S Z 1999 Naturforsch. B. Chem. Sci. 54b 1175
Näther C and Jess I 2003 Inorg. Chem. 42 2968
Acknowledgements
The work is a part of Ph.D. dissertation of Jency Thomas submitted at Indian Institute of Technology, Delhi, India. A R acknowledges DST, Government of India, for financial support as well as powder and single crystal X-ray diffraction facility to the Department of Chemistry, IIT Delhi, India.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information (SI)
Crystallographic information files (CIF) for 1–9; crystal structure description of 1–9; figures showing weak interactions in 1–9; simulated and experimental powder XRD (Figures S8–S15); á posteriori analysis of solids reported in literature with pyridazine, pyrimidine and pyrazine (Figures S16–S23); results of rietveld refinement for phase quantification of orthorhombic and triclinic phases in 6 and 7 (Figure S24); results of vibrational and thermal analysis (Figures S25–S28); crystallographic details for solids 1–9 (Table S5) are given in the Supplementary Information available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
THOMAS, J., RAMANAN, A. What Triggers Supramolecular Isomerism in Nonmolecular Solids? A case study of Copper Pyridazine Halides. J Chem Sci 128, 1687–1694 (2016). https://doi.org/10.1007/s12039-016-1179-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1179-9