Abstract
Computational study of X-H⋯C and C-H⋯X hydrogen bonds in n-alkane-HX complexes (X =F,OH, alkane =propane, butane, pentane) has been carried out in this work. Ab initio and density functional theories were used for this study. For n-alkane-H2O complexes both O⋯H-C and O–H⋯C hydrogen bonded complex have been found, while for n-alkane-HF complexes, our attempt to optimize F⋯H-C H-bond was not successful. Like most of the hydrogen bonded systems, strong correlation between binding energy and stretching frequency of H-F and O-H stretching mode was observed. The values of electron density and Laplacian of electron density are within the accepted range for hydrogen bonds. In all these cases, X-H⋯C hydrogen bonds are found to be stronger than C-H⋯X hydrogen bonds.

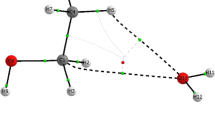
O–H⋯C hydrogen bond in n-butane represents n-alkane-HX (X = F, OH) complexes. X-H⋯C hydrogen bonds are found to be stronger than C-H⋯X hydrogen bonds.







Similar content being viewed by others
References
Szczçśniak M M, Chałasiński G, Cybulski S M and Cieplak P 1993 J. Chem. Phys. 98 3078
Novoa J J and Tarron B 1991 J. Chem. Phys. 95 5179
Rovira C and Novoa J J 1997 Chem. Phys. Lett. 279 140
Kozmutza C, Varga I and Udvardi L 2003 Theochem 666–667 95
Hartman M, Wetmore S D and Radom L 2001 J. Phys. Chem. A 105 4470
Novoa J J, Planas M and Carme Rovira M 1996 Chem. Phys. Lett. 251 33
Ojo O A and Szalewicz K 2005 J. Chem. Phys. 123 134311
Suenram R D, Fraser G T, Lovas F J and Kawashima Y J 1994 Chem. Phys. 101 7230
Raghavendra B and Arunan E 2008 Chem. Phys. Lett. 467 37
Pauling L 1960 In The Nature of the Chemical Bond and the Structure of Molecules and Crystals, An Introduction to Modern Structural Chemistry (NY: Cornell University Press Ithaca) p 478
Raghavendra B and Arunan E 2007 J. Phys. Chem. A 111 9699
Hammerum S 2009 J. Am. Chem. Soc. 131 8627
Arunan E, Desiraju G R, Klein R A, Sadlej J, Scheiner S, Alkorta I, Clary D C, Crabtree R H, Dannenberg J J, Hobza P, Kjaergaard H G, Legon A C, Mennucci B and Nesbitt D J 2011 Pure Appl. Chem. 83 1619
Arunan E, Desiraju G R, Klein R A, Sadlej J, Scheiner S, Alkorta I, Clary D C, Crabtree R H, Dannenberg J J, Hobza P, Kjaergaard H G, Legon A C, Mennucci B and Nesbitt D J 2011 Pure Appl. Chem. 83 1637
Davis S R and Andrews L 1987 J. Am. Chem. Soc. 109 4768
Bonaccorsi R, Scrocco E and Tomasi J 1970 J. Chem. Phys. 52 5270
Politzer P 1981 In Chemical Applications of Atomic and Molecular Electrostatic Potential D G Truhlar (ed.) (New York: Plenum), pp. 1–6
Gadre S R and Shirsat R N 2000 In Electrostatics of Atoms and Molecules (Hyderabad: Universities Press (India) Ltd.) p. 63
Politzer P, Murray J S and Clark T 2010 Phys. Chem. Chem. Phys. 12 7748
Gadre S R and Bhadane P K 1997 J. Chem. Phys. 107 5625
Gadre S R and Pathak R K 1990 Proc. Indian Acad. Sci. (J. Chem. Sci.) 102 189
Chandra A K, Pal S, Limaye A C and Gadre S R 1995 Chem. Phys. Lett. 247 95
Gadre S R and Pingale S S 1998 J. Am. Chem. Soc. 120 7056
Gadre S R and Bhadane P K 1998 Theor. Chem. Acc. 100 300
Mani D and Arunan E 2013 Phys. Chem. Chem. Phys. 15 14377
Ambrosetti A, Costanzo F and Silvestrelli P L 2011 J. Phys. Chem. C 115 12121
Olesen S G and Hammerum S 2009 J. Phys. Chem. A 113 7940
Bader R F W 1990 In Atoms in Molecules: A Quantum Theory (Oxford: Clarendon Press)
Koch U and Popelier P L A 1995 J. Phys. Chem. 99 9747
Chr M. and Plesset M S 1934 Phys. Rev. 46 618
Pople J A, Raghavachari K, Schlegel H B and Binkley J S 1979 Int. J. Quantum Chem., Quant. Chem. Symp. S13 225
Head-Gordon M, Pople J A and Frisch M J 1988 Chem. Phys. Lett. 153 503
Becke A D 1993 J. Chem. Phys. 98 5648
Lee C, Yang W and Parr R 1988 Phys. Rev. B 37 785
Stephens P J, Devlin F J, Chabalowski C F and Frisch M J 1994 J. Phys. Chem. 98 11623
Frisch M J, Pople J A and Stephen Binkley J 1984 J. Chem. Phys. 80 3265
Zhao Y, Schultz N E and Truhlar D G 2006 J. Chem. Theory Comput. 2 364
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A J., Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara K, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A, Gaussian 03, revision E.01, Gaussian, Inc, Wallingford, CT (2004)
Boys S B and Bernardi F 1970 Mol. Phys. 19 553
Biegler-Konig F, Schonbohm J, Derdau R, Bayles D and Bader R F W, AIM 2000, version 1, Büro für Innovative Software, Bielefeld, Germany (2000)
Dennington R, Keith T and Millam J, GaussView, Version 5, Semichem Inc., Shawnee Mission KS (2009)
Bader R F W, Carroll M T, Cheeseman J R and Chang C 1987 J. Am. Chem. Soc. 109 7968
Lu T and Chen F 2012 J. Comput. Chem. 33 580
Huang Z Z and Miller R E 1988 J. Phys. Chem. 92 46
Merrit J M, Rudic S and Miller R E 2006 J. Chem. Phys. 124 084301
Bulychev V P, Gromova E I and Tokhadge K G 2004 Opt. Spectrosc. 96 774
Sousa S F, Fernandes P A and Ramos M J 2007 J. Phys. Chem. A 111 10439
Shahi A and Arunan E 2014 Phys. Chem. Chem. Phys. 16 22935
Acknowledgements
RP thanks Academy of Science for Developing World for TWAS-UNESCO Associateship, Prof. Rangarajan, the then Chairman, International Relation Cell, Indian Institute of Science and all the lab-members of Prof. Arunan’s laboratory. We thank the Supercomputer Education Research Centre at the Indian Institute of Science for computational facilities. RP would like to acknowledge Prof E Krishnakumar and Tata Institute of Fundamental Research (TIFR), Mumbai, India, for the visiting scientist position in January 2013 and January 2015 where the final form of this manuscript was prepared and revised. RP also acknowledges Centre for Science and Technology of the Non-Aligned and Other Developing Countries (NAM S&T Centre), New Delhi, for providing travel grants to visit TIFR in January 2015 and Dr. Leela Pradhan Joshi, Department of Physics, Amrit Campus, Tribhuvan University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Supplementary information is available at www.ias.ac.in/chemsci. In table S1, coordinate of propane optimized at B3LYP/6-311 ++g** is given. Positions of maximum and minimum values of ESPs and the values of ESPs are given in S2. The corresponding values for butane and pentane are given in tables S3, S4, S5 and S6, respectively. Some relevant parameters optimized with M05-2X/6-311 ++G** level of theory of the complex are presented in tables S7 and S8. Penetration parameters, bonding radius of acceptor, non-bonding radius of hydrogen atom, bonding radius of hydrogen atom are given in table S9. Changes in atomic volumes, populations, and energies are given in tables S10, S11 and S12, respectively. In table S13, changes in atomic first moments of the “H” of F-H⋯alkane complexes are given. The optimized structure of Water⋯alkane complex at B3LYP/6-311 ++g** level of theory is given in figure S1.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
PARAJULI, R., ARUNAN, E. X-H⋯C hydrogen bonds in n-alkane-HX (X = F, OH) complexes are stronger than C-H⋯X hydrogen bonds. J Chem Sci 127, 1035–1045 (2015). https://doi.org/10.1007/s12039-015-0861-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0861-7