Abstract
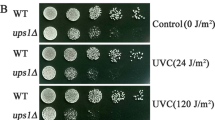
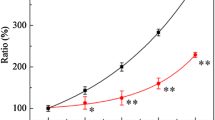
The present study aims to investigate the role of radiation sensitive 52 (RAD52) and high-affinity DNA binding factor 1 (HDF1) DNA repair genes on the life span of budding yeasts during chronological aging. Wild type (wt) and rad52, hdf1, and rad52 hdf1 mutant Saccharomyces cerevisiae strains were used. Chronological aging and survival assays were studied by clonogenic assay and drop test. DNA damage was analyzed by electrophoresis after phenol extraction. Mutant analysis, colony forming units and the index of respiratory competence were studied by growing on dextrose and glycerol plates as a carbon source. Rad52 and rad52 hdf1 mutants showed a gradual decrease in surviving fraction in relation to wt and hdf1 mutant during aging. Genomic DNA was spontaneously more degraded during aging, mainly in rad52 mutants. This strain showed an increased percentage of revertant colonies. Moreover, all mutants showed a decrease in the index of respiratory competence during aging. The inactivation of RAD52 leads to premature chronological aging with an increase in DNA degradation and mutation frequency. In addition, RAD52 and HDF1 contribute to maintain the metabolic state, in a different way, during chronological aging. The results obtained could have important implications in the chronobiology of aging.




Similar content being viewed by others
References
Allen JF 1996 Separate sexes and the mitochondrial theory of ageing. J. Theor. Biol. 180 135–140
Aung-Htut MT, Ayer A, Breitenbach M and Dawes IW 2012 Oxidative stresses and ageing; in Aging research in yeast, subcellular biochemistry 57 (eds) M. Breitenbach, SM Jazwinski, P Laun (London: Springer Science+Bussiness Media BV) pp 13–54
Baker GT and Sprott RL 1988 Biomarkers of aging. Exp. Gerontol. 23 223–239
Beach A, Leonov A, Arlia-Ciommo A, Svistkova V, Lutchman V and Titorenko VI 2015 Mechanisms by which different functional states of mitochondria define yeast longevity. Int. J. Mol. Sci. 16 5528–5554
Breitenbach M, Laun P and Jazwinski M 2012 Introduction; in Aging research in yeast, subcellular biochemistry 57 (eds) M. Breitenbach, SM Jazwinski, P Laun (London: Springer Science+Bussiness Media BV) pp 1–12
Bryan TM, Englezou A, Dunham MA and Reddel RR 1998 Telomere length dynamics in telomerase–positive immortal human cell populations. Exp. Cell Res. 239 370–378
Burhans WC and Heintz NH 2009 The cell cycle is a redox cycle: linking phase-specific targests to cell fate. Free Radic. Biol. Med. 47 1282–1293
Burhans WC and Weinberger M 2012 DNA damage and DNA replication stress in yeast models of aging; in Aging research in yeast, subcellular biochemistry 57 (eds) M. Breitenbach, SM Jazwinski, P Laun (London: Springer Science+Bussiness Media BV) pp 187–206
Busuttil RA, Dolle M, Campisi J and Vijga J 2004 Genomic instability aging, and cellular senescence. Ann. N. Y. Acad. Sci. 1019 245–255
Busuttil RA, Garcia AM, Cabrera C, Rodriguez A, Suh Y, Kim WH, Huang TT and Vijg J 2005 Organ-specific increase in mutation accumulation and apoptosis rate in CuZn-superoxide dismutase–deficient mice. Cancer Res. 65 11271–11275
Chen BR and Runge KW 2012 Genetic approaches to aging in budding and fission yeasts: new connections and new opportunities; in Aging research in yeast, subcellular biochemistry 57 (eds) M. Breitenbach, SM Jazwinski, P Laun (London: Springer Science+Bussiness Media BV) pp 291–314
Coppedè F and Migliore L 2010 DNA repair in premature aging disorders and neurodegeneration. Curr. Aging Sci. 3 3–19
De Boer J, Andressoo JO, De Wit J, Huijmans J, Beems RB, Van Steeg H, Weeda G, Van der Horst GT, et al. 2002 Premature aging in mice deficient in DNA repair and transcription. Science 296 1276–1279
Di Primio C, Galli A, Cervelli T, Zoppè M and Rainaldi G 2005 Potentiation of gene targeting in human cells by expression of Saccharomyces cerevisiae Rad52. Nucleic Acids Res. 33 4639–4648
Diderich KE, Nicolaije C, Priemel M, Waarsing JH, Day JS, Brandt RM, Schilling AF, Botter SM, et al. 2012 Bone fragility and decline in stem cells in prematurely aging DNA repair deficient trichothiodystrophy mice. Age 34 845–861
Fabrizio P and Longo VD 2003 The chronological life span of Saccharomyces cerevisiae. Aging Cell 2 73–81
Fabrizio P and Longo VD 2008 Chronological aging-induced apoptosis in yeast. Biochim. Biophys. Acta 1783 1280–1285
Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, Dossen JW, Gralla EB and Longo VD 2004a Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J. Cell Biol. 166 1055–1067
Fabrizio P, Pletcher SD, Minois N, Vaupel JW and Longo VD 2004b Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 557 136–142
Fabrizio P, Gattazzo C, Battistella L, Wi M, Cheng, McGrew, K and Longo VD 2005a Sir2 blocks extreme life-span extension. Cell 123 655–667
Fabrizio P, Li L and Longo VD 2005b Analysis of gene expression profile in yeast aging chronologically. Mech. Ageing Dev. 126 11–16
Friedl AA, Kiechle M, Fellerhoff B and Eckardt-Schupp F 1998 Radiation-induced chromosome aberrations in Saccharomyces cerevisiae: Influence of DNA repair pathways. Genetics 148 975–988
Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, Büttner S, Fehr M, Sigrist S, Madeo F 2004 Chronological aging leads to apoptosis in yeast. J. Cell Biol. 164 501–507
Hoffman CS and Winston F 1987 A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57 267–272
Kaya A, Lobanov AV and Gladyshev VN 2015 Evidence that mutations accumulation does not cause aging in Saccharomyces cerevisiae. Aging Cell 14 366–371
Kitanovic A and Wölfl S 2006 Fructose-1,6-biphosphatase mediates cellular responses to DNA damage and aging in Saccharomyces cerevisiae. Mutat. Res. 594 135–147
Laqué-Rupérez E, Ruiz-Gómez MJ, de la Peña L, Gil L and Martínez-Morillo M 2003 Methotrexate cytotoxicity on MCF-7 breast cancer cells is not altered by exposure to 25 Hz, 1.5 mT magnetic field and iron (III) chloride hexahydrate. Bioelectrochemistry 60 81–86
Laschober GT, Ruli D, Hofer E, Muck C, Carmona-Gutierrez D, Ring J, Hutter E, Ruckenstuhl C, et al. 2010 Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell 9 1084–1097
Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang J-D, Li KM, et al. 2005 Genomic instability in laminopathy-based premature aging. Nat. Med. 11 780–785
Ljunqquist B, Berg S, Lanke J, McClearn GE and Pedersen NL 1998 The effects of genetic factors for longevity: A comparison of identical and fraternal twins in the Swedish Twin Registry. J. Gerontol. Med. Sci. 53 441–446
Longo VD and Fabrizio P 2012 Chronological aging in Saccharomyces cerevisiae; in Aging research in yeast, subcellular biochemistry 57 (eds) M. Breitenbach, SM Jazwinski, P Laun (London: Springer Science+Bussiness Media BV) pp 101–121
López-Díaz B, Mercado-Sáenz S, Martínez-Morillo M, Sendra-Portero F and Ruiz-Gómez MJ 2014 Long-term exposure to a pulsed magnetic field (1.5 mT, 25 Hz) increases genomic DNA spontaneous degradation. Electromagn. Biol. Med. 33 228–235
Lynch M, Sung W, Morris K, Coffey N, Landry CR, Dopman EB, Dickinson WJ, Okamoto K, et al. 2008 A genome-wide view of the spectrum of spontaneous mutations in yeast. Proc. Natl. Acad. Sci. U. S. A. 105 9272–9277
Maclean M, Aamodt R, Harris N, Alseth I, Seeberg E, Bjørås M and Piper PW 2003 Base excision repair activities required for yeast to attain a full chronological life span. Aging Cell 2 93–104
Madeo F, Frohlich E, Ligr M, Grey M, Sigrist SJ, Wolf DH and Frohlich KU 1999 Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145 757–767
Mbantenkhu M, Wang X, Nardozzi JD, Wilkens S, Hoffman E, Patel A, Cosgrove MS, and Chen XJ 2011 Mgm101 is a Rad52-related protein required for mitochondrial DNA recombination. J. Biol. Chem. 286 42360–42370
McGue M, Vaupel JW, Holm N and Harvald B 1993 Longevity is moderately heritable in a sample of Danish twins born 1870–1880. J. Gerontol. Biol. Sci. 48 237–244
McMurray MA and Gottschling DE 2004 Aging and genetic instability in yeast. Curr. Opin. Microbiol. 7 673–679
Mercado-Sáenz S, Ruiz-Gómez MJ, Morales-Moreno F and Martínez-Morillo M 2010 Cellular aging: theories and technological influence. Braz. Arch. Biol. Technol. 53 1319–1332
Milne GT, Jin S, Shannon KB and Weaver DT 1996 Mutations at two ku homologs define a DNA end-joining repair pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 16 4189–4198
Muid KA, Karakaya HC and Koc A 2014 Absence of superoxide dismutase activity causes nuclear DNA fragmentation during the aging process. Biochem. Biophys. Res. Commun. 444 260–263
Park PU, Defossez PA and Guarente L 1999 Effects of mutations in DNA repair genes on formation of ribosomal DNA circles and life span in Saccharomyces cerevisiae. Mol. Cell. Biol. 19 3848–3856
Parrella E and Longo VD 2008 The chronological life span of Saccharomyces cerevisiae to study mitochondrial dysfunction and disease. Methods 46 256–262
Potenza L, Cucchiarini L, Piatti E, Angelini U and Dachà M 2004 Effects of high static magnetic field exposure on different DNAs. Bioelectromagnetics 25 352–355
Qin H and Lu M 2006 Natural variation in replicative and chronological life spans of Saccharomyces cerevisiae. Exp. Gerontol. 41 448–456
Ruiz-Gómez MJ and Martínez-Morillo M 2005 Enhancement of the cell-killing effect of ultraviolet-C radiation by short-term exposure to a pulsed magnetic field. Int. J. Radiat. Biol. 81 483–490
Ruiz-Gómez MJ, Pastor Vega JM, de la Peña L, Gil Carmona L and Martínez Morillo M 1999 Growth modification of human colon adenocarcinoma cells exposed to a low-frequency electromagnetic field. J. Physiol. Biochem. 55 79–84
Ruiz-Gómez MJ, Merino-Moyano MD, Cebrián-Martín MG, Prieto-Barcia MI and Martínez-Morillo M 2008 No effect of 50 Hz 2.45mT magnetic field on the potency of cisplatin, mitomycin C, and methotrexate in S. cerevisiae. Electromagn. Biol. Med. 27 289–297
Ruiz-Gómez MJ, Ristori-Bogajo E, Prieto-Barcia MI and Martínez-Morillo M 2010a No evidence of cellular alterations by milliTesla-level static and 50Hz magnetic fields on S. cerevisiae. Electromagn. Biol. Med. 29 154–164
Ruiz-Gómez MJ, Sendra-Portero F and Martínez-Morillo M 2010b Effect of 2.45 mT sinusoidal 50Hz magnetic field on Saccharomyces cerevisiae strains deficient in DNA strand breaks repair. Int. J. Radiat. Biol. 86 602–611
Umezu K, Sugawara N, Chen C, Haber JE and Kolodner RD 1998 Genetic analysis of yeast RPA1 reveals its multiple functions in DNA metabolism. Genetics 148 989–1005
Warner HR, Butler RN, Richard L and Sprott PD 1987 Modern biological theories of aging (New York: Raven Press)
Wauters T, Iserentant D and Verachtert H 2001 Impact of mitochondrial activity on the cell wall composition and on the resistance to tannic acid in Saccharomyces cerevisiae. J. Gen. Appl. Microbiol. 47 21–26
Acknowledgements
We express our gratitude to Dr Anna A Friedl (Department of Radiation Oncology, Ludwig-Maximilians-Universität München, Munich, Germany) for kindly providing the yeast strains. We also thank Ms L Gil Carmona (Universidad de Málaga, Spain) for her technical assistance.
This study was supported by the ‘Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI); Junta de Andalucía’, [CTS-181].
Author information
Authors and Affiliations
Corresponding author
Additional information
† In memoriam.
[Mercado-Sáenz S, López-Díaz B, Sendra-Portero F, Martínez-Morillo M and Ruiz-Gómez MJ 2017 Inactivation of RAD52 and HDF1 DNA repair genes leads to premature chronological aging and cellular instability. J. Biosci.]
Rights and permissions
About this article
Cite this article
Mercado-Sáenz, S., López-Díaz, B., Sendra-Portero, F. et al. Inactivation of RAD52 and HDF1 DNA repair genes leads to premature chronological aging and cellular instability. J Biosci 42, 219–230 (2017). https://doi.org/10.1007/s12038-017-9684-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-017-9684-7