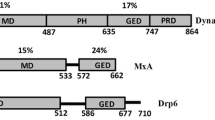
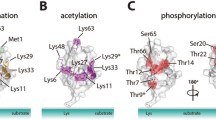
Abstract
Dynamin superfamily proteins comprising classical dynamins and related proteins are membrane remodelling agents involved in several biological processes such as endocytosis, maintenance of organelle morphology and viral resistance. These large GTPases couple GTP hydrolysis with membrane alterations such as fission, fusion or tubulation by undergoing repeated cycles of self-assembly/disassembly. The functions of these proteins are regulated by various post-translational modifications that affect their GTPase activity, multimerization or membrane association. Recently, several reports have demonstrated variety of such modifications providing a better understanding of the mechanisms by which dynamin proteins influence cellular responses to physiological and environmental cues. In this review, we discuss major post-translational modifications along with their roles in the mechanism of dynamin functions and implications in various cellular processes.

Similar content being viewed by others
Abbreviations
- CaMKIα:
-
calmodulin-dependent protein kinase 1alpha
- Cav1:
-
Caveolin1
- CDK:
-
cyclin-dependent kinase
- Drp:
-
dynamin-related protein
- eNOS:
-
endothellial nitric oxide synthase
- ER:
-
endoplasmic reticulum
- ERK:
-
extracellular regulated kinase
- GED:
-
GTPase effector domain
- GTP:
-
guanosine triphosphate
- HDAC:
-
histone deacetylase
- JNK:
-
Janus kinase
- MAM:
-
mitochondria-associated ER membrane
- MD:
-
middle domain
- Mfn:
-
mitofusin
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase
- OMM:
-
outer mitochondrial membrane
- PHD:
-
Pleckstrin homology domain
- PKA:
-
protein kinase A
- PKC:
-
protein kinase C
- PRD:
-
proline-rich domain
- ROS:
-
reactive oxygen species
- SH3:
-
Src homology 3
- SIRT:
-
sirtuin
References
Ahn S, Kim J, Lucaveche CL, Reedy MC, Luttrell LM, Lefkowitz RJ and Daaka Y 2002 Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 277 26642–26651.
Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, et al. 2000 OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 26 211–215.
Altmann K and Westermann B 2005 Role of essential genes in mitochondrial morphogenesis in Saccharomyces cerevisiae. Mol. Biol. Cell. 16 5410–5417.
Anggono V, Smillie KJ, Graham ME, Valova VA, Cousin MA and Robinson PJ 2006 Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat. Neurosci. 9 752–760
Anton F, Dittmar G, Langer T and Escobar-Henriques M 2013 Two deubiquitylases act on mitofusin and regulate mitochondrial fusion along independent pathways. Mol. Cell. 49 487–498
Armbruster M, Messa M, Ferguson SM, De Camilli P and Ryan TA 2013 Dynamin phosphorylation controls optimization of endocytosis for brief action potential bursts. eLife. doi:10.7554/eLife.00845
Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J and Frolov VA 2008 GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 135 1276–1286
Bitoun M, Maugenre S, Jeannet P-Y, Lacène E, Ferrer X, Laforêt P, Martin J-J, Laporte J, et al. 2005 Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 37 1207–1209
Bitoun M, Bevilacqua JA, Eymard B, Prudhon B, Fardeau M, Guicheney P and Romero NB 2009 A new centronuclear myopathy phenotype due to a novel dynamin 2 mutation. Neurology. 72 93–95
Bohren KM, Nadkarni V, Song JH, Gabbay KH and Owerbach D 2004 A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J. Biol. Chem. 279 27233–38
Brantis-de-Carvalho CE, Maarifi G, Boldrin PEG, Zanelli CF, Nisole S, Chelbi-Alix MK and Valentini SR 2015 MxA interacts with and is modified by the SUMOylation machinery. Exp. Cell Res. 330 151–163
Broillet MC 1999 S-nitrosylation of proteins. Cell. Mol. Life Sci. 55 1036–1042
Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC and Shah V 2001 Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J. Biol. Chem. 276 14249–14256
Carr JF and Hinshaw JE 1997 Dynamin assembles into spirals under physiological salt conditions upon the addition of GDP and ??-phosphate analogues. J. Biol. Chem. 272 28030–28035
Cereghetti GM, Stangherlin A, de Brito OM, Chang CR, Blackstone C, Bernardi P and Scorrano L 2008 Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. USA 105 15803–15808
Chan DC 2006 Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 22 79–99
Chang C-R and Blackstone C 2007 Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 282 21583–21587
Chang CR and Blackstone C 2010 Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. in Annals of the New York Academy of Sciences 1201 34–39
Chen H and Chan DC 2005 Emerging functions of mammalian mitochondrial fusion and fission. Hum. Mol. Genet. 14 R283–289
Chen Y and Dorn GW 2013 PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science (New York, N.Y.) 340 471–475
Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC and Vallee RB 1991 Multiple forms of dynamin are encoded by shibire, a drosophila gene involved in endocytosis. Nature 351 583–586
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE and Chan DC 2003 Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and Are essential for embryonic development. J. Cell Biol. 160 189–200
Cho D-HH, Nakamura T, Fang J, Cieplak P, Godzik A, Zezong G and Lipton SA 2009 S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324 102–105
Cho B, Cho HM, Kim HJ, Jeong J, Park SK, Hwang EM, Park J-Y, Kim WR, et al. 2014 CDK5-dependent inhibitory phosphorylation of Drp1 during neuronal maturation. Exp. Mol. Med. 46, e105
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV and Mann M 2009 Lysine acetylation targets protein complexes and Co-regulates major cellular functions. Science 325 834–840
Choudhary C, Weinert BT, Nishida Y, Verdin E and Mann M 2014 The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15 536–550
Cocucci E, Gaudin R and Kirchhausen T 2014 Dynamin recruitment and membrane scission at the neck of a clathrin-coated pit. Mol. Biol. Cell. 25 3595–3609
David C, McPherson PS, Mundigl O and de Camilli P 1996 A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc. Natl. Acad. Sci. USA 27293 331–335
Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, Nicols PP, Boulton ME, et al. 2007 Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum. Mol. Genet. 16 1307–13018
de Brito OM and Scorrano L 2008 Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456 605–610
Durieux A-C, Prudhon B, Guicheney P and Bitoun M 2010 Dynamin 2 and human diseases. J. Mol. Med. 88 339–350
Ekena K, Vater CA, Raymond CK and Stevens TH 1993 The VPS1 protein is a dynamin-like GTPase required for sorting proteins to the yeast vacuole. Ciba Found. Symp. 176 198–211
Estela A-G, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof Ad J , Madra M, Ikenouchi J, Umeda M, et al. 2012 Upregulated function of mitochondria-associated {ER} membranes in Alzheimer disease. EMBO J. 31 4106–4123
Eura Y, Ishihara N, Yokota S and Mihara K 2003 Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J. Biochem. 134 333–344
Figueroa-Romero C, Iñiguez-Lluhí JA, Stadler J, Chang C-R, Arnoult D, Keller PJ, Hong Y, Blackstone C, and Feldman EL 2009 SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J. 23 3917–3927
Fish KN, Schmid SL and Damke H 2000 Evidence that dynamin-2 functions as a signal-transducing GTPase. J. Cell Biol. 150 145–154
Fisk HA and Yaffe MP 1999 A role for ubiquitination in mitochondrial inheritance in Saccharomyces cerevisiae. J. Cell Biol. 145 1199–1208
Fritz S, Weinbach N and Westermann B 2003 Mdm30 is an F-box protein required for maintenance of fusion-competent mitochondria in yeast. Mol. Biol. Cell 14 2303–2313
Gad H, Ringstad N, Löw P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, et al. 2000 Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 27 301–312.
Gao S, von der Malsburg A, Paeschke S, Behlke J, Haller O, Kochs G and Daumke O, Alexander von der Malsburg, Susann Paeschke, Joachim Behlke, Otto Haller, Georg Kochs, and Oliver Daumke. 2010 Structural basis of oligomerization in the stalk region of dynamin-like MxA. Nature 465 502–506
Gastaldelli M, Imelli N, Boucke K, Amstutz K, Meier O and Greber U F Greber 2008 Infectious adenovirus type 2 transport through early but not late endosomes. Traffic 9 2265–2278
Gould N, Doulias PT, Tenopoulou M, Raju K and Ischiropoulos H 2013 Regulation of protein function and signaling by reversible cysteine s-nitrosylation. J. Biol. Chem. 288 26473–26479
Guo C, Hildick KL, Luo J, Dearden L, Wilkinson KA, Henley JM, Anderson DB, Wilkinson KA and Henley JM 2013 SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J. 32 1514–1528. doi:10.1038/emboj.2013.65
Han X-JJ, Lu Y-FF, Li S-AA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H and Matsushita M 2008 {CaM} kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell Biol. 182 573–585
Hao G et al. 2006 SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc. Natl. Acad. Sci. USA 103 1012–1017
Harder Z, Zunino R and McBride H 2004 Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr. Biol. 14 340–345
Haun F, Nakamura T, Shiu AD, Cho D-H, Tsunemi T, Holland EA, La Spada AR and Lipton SA 2013 S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington’s disease. Antioxid. Redox Signal 19 1173–1184
Hedskog L, Pinho CM, Filadi R, Rönnbäck A, Hertwig L, Wiehager B, Larssen P, Gellhaar S, et al. 2013 Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc. Natl. Acad. Sci. USA 110 7916–7921
Hill E, Van Der Kaay J, Downes CP and Smythe E 2001 The role of dynamin and its binding partners in coated pit invagination and scission. J. Cell Biol. 152 309–323
Hinshaw JE 2000 Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 16 483–519
Humbert S, Dhavan R and Tsai L 2000 P39 activates Cdk5 in neurons, and is associated with the actin cytoskeleton. J. Cell Sci. 113 975–983
Ishihara N, Eura Y and Mihara K 2004 Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci. 117 6535–6546
Jahani-Asl A, Huang E, Irrcher I, Rashidian J, Ishihara N, Lagace DC, Slack RS and Park DS 2015 CDK5 phosphorylates DRP1 and drives mitochondrial defects in NMDA-induced neuronal death. Hum. Mol. Genet. 24 4573–4583
Janzen C, Kochs G and Haller O 2000 A monomeric GTPase-negative MxA mutant with antiviral activity. J. Virol. 74 8202–8206
Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM and Kahn CR 2011 Sirtuin-3 (Sirt3) Regulates Skeletal Muscle Metabolism and Insulin Signaling via Altered Mitochondrial Oxidation and Reactive Oxygen Species Production. Proc. Natl. Acad. Sci. USA 108 14608–14613
Kang-Decker, Ningling, Sheng Cao, Suvro Chatterjee, Janet Yao, Laurence J Egan, David Semela, Debabrata Mukhopadhyay, andVijay Shah 2007 Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J. Cell Sci. 120 492–501
Karbowski M, Neutzner A and Youle RJ 2007 The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J. Cell Biol. 178 71–84
Kim Y and Chang S 2006 Ever-expanding network of dynamin-interacting proteins. Mol. Neurobiol. 34 129–136
Kim HJ, Nagano Y, Choi SJ, Park SY, Kim H, Yao TP and Lee JY 2015 HDAC6 maintains mitochondrial connectivity under hypoxic stress by suppressing MARCH5/MITOL dependent MFN2 degradation. Biochem. Biophys. Res. Commun. 464 1235–1240
Knott AB and Bossy-Wetzel E 2008 Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. in Annals of the New York Academy of Sciences pp. 283–292
Komander D and Rape M 2012 The ubiquitin code. Annu. Rev. Biochem. 81 203–229
Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM and Chan DC 2004 Structural basis of mitochondrial tethering by mitofusin complexes. Science (New York, N.Y.) 305 858–862
Kuravi K, Nagotu S, Krikken AM, Sjollema K, Deckers M, Erdmann R, Veenhuis M and van der Klei IJ 2006 Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J. Cell Sci. 119 3994–4001
Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, Veenstra TM, Glickman MH and Weissman AM 2012 Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol. Cell 47 547–557
Lee JE, Westrate LM, Wu H, Page C and Voeltz G 2016 Multiple dynamin family members collaborate to drive mitochondrial division. Nature 540 139–143
Liesa M, Palacín M and Zorzano A 2009 Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 89 799–845
Liu Y-W, Neumann S, Ramachandran R, Ferguson SM, Pucadyil TJ and Schmid SL 2011 Differential curvature sensing and generating activities of dynamin isoforms provide opportunities for tissue-specific regulation. Proc. Natl. Acad. Sci. USA 108 E234–E242
Liu YW, Mattila JP and Schmid SL 2013 Dynamin-catalyzed membrane fission requires coordinated GTP hydrolysis. PLoS ONE. 8
Meier O and Greber UF 2003 Adenovirus endocytosis. J. Gene Med. 5 451–462
Montessuit S, Somasekharan SP, Terrones O,Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, et al. 2010 Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 142 889–901
Nakamura N, Kimura Y, Tokuda M, Honda S and Hirose S 2006 MARCH-V} is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep. 7 1019–1022
Nakamura T, Cieplak P, DH Cho, Godzik A and Lipton SA 2010 S-Nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion 10 573–578
Nannapaneni S, Wang D, Jain S, Schroeder B, Highfill C, Reustle L, Pittsley D, Maysent A, et al. 2010 The yeast dynamin-like protein Vps1:vps1 mutations perturb the internalization and the motility of endocytic vesicles and endosomes via disorganization of the actin cytoskeleton. Eur. J. Cell Biol. 89 499–508
Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE and Ryan MT 2011 {MiD49} and {MiD51,} new components of the mitochondrial fission machinery. EMBO Rep. 12 565–573
Park Y-Y and Cho H 2012 Mitofusin 1 is degraded at G2/M phase through ubiquitylation by MARCH5. Cell Div. 7 25
Park Y-Y, Lee S, Karbowski M, Neutzner A, Youle RJ and Cho H 2010 Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J. Cell Sci. 123 619–626
Park, Y-Y, OTK Nguyen, H Kang, and H Cho 2014 MARCH5-mediated quality control on acetylated Mfn1 facilitates mitochondrial homeostasis and cell survival. Cell Death Dis. 5 e1172
Pichler A, Gast A, Seeler JS, Dejean A and Melchior F 2002 The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108 109–120
Pitts KR, Yoon Y, Krueger EW and McNiven MA 1999 The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol. Biol. Cell. 10 4403–4417
Praefcke GJK and McMahon HT 2004 The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell. Biol. 5 133–147
Pucadyil TJ and Schmid SL 2008 Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell 135 1263–1275
Pyakurel A, Savoia C, Hess D and Scorrano L 2015 Extracellular regulated kinase phosphorylates mitofusin 1 to control mitochondrial morphology and apoptosis. Mol. Cell 58 244–254
Qi X, Disatnik M-H, Shen N, Sobel RA, and Mochly-Rosen D 2011 Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol. Biol. Cell 22 256–265
Qualmann B and Kelly RB 2000 Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 148 1047–1061
Rahaman A, Elde NC and Turkewitz AP 2008 A dynamin-related protein required for nuclear remodeling in tetrahymena. Curr. Biol. 18 1227–1233
Reid KSC, Lindley PF and Thornton JM 1985 Sulphur-aromatic interactions in proteins. FEBS Lett. 190 209–213
Reubold TF et al. 2005 Crystal structure of the GTPase domain of rat dynamin 1. Proc. Natl. Acad. Sci. USA 102 13093–13098
Robinson PJ, Sontag JM, Liu JP, Fykse, C Slaughter EM, McMahon H and Südhof TC 1993 Dynamin GTPase regulated by protein kinase C phosphorylation in nerve terminals. Nature 365 163–166
Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC and Gupta MP 2014 SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol. Cell. Biol. 34 807–819
Sever S, Damke H and Schmid SL 2000 Dynamin: GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J. Cell Biol. 150 1137–1147
Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB and Minshall RD 2004 Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 279 20392–20400
Shpetner HS and Vallee RB 1989 Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell 59 421–432
Smaczynska-de Rooij II, Marklew CJ, Allwood EG, Palmer SE, Booth WI, Mishra R, Goldberg MW and Ayscough KR 2015 Phosphorylation regulates the endocytic function of the yeast dynamin-related protein Vps1. Mol. Cell Biol. 36 742–755
Song BD, Leonard M and Schmid SL 2004 Dynamin GTPase domain mutants that differentially affect GTP binding, GTP hydrolysis, and clathrin-mediated endocytosis. J. Biol. Chem. 279 40431–40436
Strack S, Wilson TJ and Cribbs JT 2013 Cyclin-dependent kinases regulate splice-specific targeting of dynamin-related protein 1 to microtubules. J. Cell Biol. 201 1037–1051
Su HL and Li SSL 2002 Molecular features of human ubiquitin-like SUMO genes and their encoded proteins. Gene. 296 65–73
Su SC and Tsai L-H 2011 Cyclin-dependent kinases in brain development and disease. Annu. Rev. Cell Dev. Biol. 27 465–491
Sugiura A, Nagashima S, Tokuyama T, Amo T, Matsuki M, Ishido S, Kudo Y, McBride HM, et al. 2013 MITOL regulates endoplasmic reticulum-mitochondria contacts via Mitofusin2. Mol. Cell 51 20–34
Taguchi N, Ishihara N, Jofuku A, Oka T, and Mihara K 2007 Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 282 11521–11529
Takei,K, Slepnev VI, Haucke V and De Camilli P 1999 Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1 33–39
Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, et al. 2003 Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol. 5 701–710
Tang J, Hu Z, Tan J, Yang S and Zeng L 2016 Parkin protects against oxygen-glucose deprivation/reperfusion insult by promoting Drp1 degradation. Oxid. Med. Cell Longev. 2016 8474303
Tsai LH, Delalle I, Caviness VS, Chae T, and Harlow E 1994 P35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371 419–423
Van der Bliek AM and Meyerowitz EM 1991 Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351 411–414
Vater CA, Raymond CK, Ekena K, Howald-Stevenson I and Stevens TH 1992 The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J. Cell Biol. 119 773–786
Wada J and Nakatsuka A 2016 Mitochondrial dynamics and mitochondrial dysfunction in diabetes. Acta Med. Okayama. 70 151–158
Wang Yonggang DM 2009 SUMOylation and deSUMOylation at a glance. J. Cell Sci. 122 4249–4252
Wang K, Huang S, Kapoor-Munshi A and Nemerow G 1998 Adenovirus internalization and infection require dynamin. J. Virol. 72 3455–3458
Wang G, Moniri NH, Ozawa K, Stamler JS and Daaka Y 2006 Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc. Natl. Acad. Sci. USA 103 1295–1300
Wang Z, Kim JII, Frilot N and Daaka Y 2012 Dynamin2 S-nitrosylation regulates adenovirus type 5 infection of epithelial cells. J. Gen. Virol. 93 2109–2117
Wasiak S, Zunino R and McBride HM 2007 Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J. Cell Biol. 177 439–450
Wilsbach K and Payne GS 1993 Vps1p, a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in Saccharomyces cerevisiae. EMBO J. 12 3049–3059
Xu S, Cherok E, Das E, Li S, Roelofs BA, SX, Polster BM, Boyman L et al. 2015 Mitochondrial E3 ubiquitin ligase MARCH5 controls mitochondrial fission and cell sensitivity to stress-induced apoptosis through regulation of MiD49 protein. Mol. Biol. Cell 27 349–359
Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, et al. 2006 A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 25 3618–3626
Youle RJ and van der Bliek AM 2012 Mitochondrial fission, fusion, and stress. Science 337 1062–1065
Yu T, Jhun BSS and Yoon Y 2010 High glucose stimulation increases reactive oxygen species production through the calcium and MAP kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal. 14 425–437
Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L et al. 2010 Regulation of cellular metabolism by protein lysine acetylation. Science (New York, N.Y.) 327 1000–1004
Züchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E et al. 2004 Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 36 449–451
Verhoeven K, Claeys K, De Jonghe P, Merory J, Oliveira SA et al. 2005 Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat. Genet. 37 289–294
Zunino R, Schauss A, Rippstein P, Miguel A-N and McBride HD 2007 The {SUMO} protease {SENP5} is required to maintain mitochondrial morphology and function. J. Cell Sci. 120 1178–1188
Acknowledgements
The work in the laboratory is supported by DBT grant (BT/PR14643/BRB/10/862/2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
[Kar UP, Dey H and Rahaman A 2017 Regulation of dynamin family proteins by post-translational modifications. J. Biosci.]
Rights and permissions
About this article
Cite this article
Kar, U.P., Dey, H. & Rahaman, A. Regulation of dynamin family proteins by post-translational modifications. J Biosci 42, 333–344 (2017). https://doi.org/10.1007/s12038-017-9680-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-017-9680-y