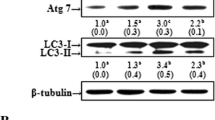
Abstract
The mediation of apoptosis-related protein in the TGF-β signaling pathway (ARTS) and X-liked inhibitor of apoptosis protein (XIAP) by parkin plays a critical role in preventing Parkinson’s disease. We studied whether carnosic acid (CA) could prevent 6-hydroxydopamine (6-OHDA)-induced apoptosis by modulating ARTS and XIAP through parkin in SH-SY5Y cells. In cells treated with 6-OHDA, the protein expression of ARTS is increased and XIAP is decreased. Pretreatment of cells with CA reversed these effects. Moreover, CA attenuated the activation of caspase 9 and caspase 7 by 6-OHDA. By immunoprecipitation with ARTS antibody, we found that 6-OHDA increased the protein expression of XIAP. However, pretreatment of cells with CA reduced XIAP protein and increased the ubiquitination of ARTS. Silencing of parkin attenuated the ability of CA to reverse the induction of ARTS and apoptotic-related proteins and the reduction of XIAP and parkin protein by 6-OHDA. Similarly, reversal of 6-OHDA-induced nuclear condensation and apoptotic-related proteins by CA was inhibited in cells with XIAP silencing. In conclusion, CA induces parkin by enhancing the ubiquitination of ARTS, leading to induction of XIAP. This may be a novel strategy for preventing Parkinson’s disease.







Similar content being viewed by others
Abbreviations
- ARTS:
-
Apoptosis-related protein in the TGF-β signaling pathway
- BIR:
-
Baculoviral IAP repeat
- CA:
-
Carnosic acid
- DMSO:
-
Dimethyl sulfoxide
- IAP:
-
Inhibitor of apoptosis family of proteins
- 6-OHDA:
-
6-Hydroxydopamine
- MPTP:
-
Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PARP:
-
Poly-ADP ribose polymerase
- PD:
-
Parkinson’s disease
- siRNA:
-
Small-interfering RNA
- UPS:
-
Ubiquitin-proteasome system
- XIAP:
-
X-liked inhibitor of apoptosis protein.
References
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535. doi:10.1016/S1474-4422(06)70471-9
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222. doi:10.1146/annurev-pathol-011110-130242
Hu LW, Yen JH, Shen YT, Wu KY, Wu MJ (2014) Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS One 9:e97880. doi:10.1371/journal.pone.0097880
Liu H, Mao P, Wang J, Wang T, Xie CH (2015) Allicin protects PC12 cells against 6-OHDA-induced oxidative stress and mitochondrial dysfunction via regulating mitochondrial dynamics. Cell Physiol Biochem 36:966–979. doi:10.1159/000430271
Liu B, Traini R, Killinger B, Schneider B, Moszczynska A (2013) Overexpression of parkin in the rat nigrostriatal dopamine system protects against methamphetamine neurotoxicity. Exp Neurol 247:359–372. doi:10.1016/j.expneurol.2013.01.001
Lin CY, Tsai CW (2016) Carnosic acid protects SH-SY5Y cells against 6-hydroxydopamine-induced cell death through upregulation of parkin pathway. Neuropharmacology 110:109–117. doi:10.1016/j.neuropharm.2016.04.017
Kazlauskaite A, Muqit MM (2015) PINK1 and parkin—mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson's disease. FEBS J 282:215–223. doi:10.1111/febs.13127
Charan RA, LaVoie MJ (2015) Pathologic and therapeutic implications for the cell biology of parkin. Mol Cell Neurosci 66:62–71. doi:10.1016/j.mcn.2015.02.008
Koch A, Lehmann-Horn K, Dachsel JC, Gasser T, Kahle PJ, Lucking CB (2009) Proteasomal inhibition reduces parkin mRNA in PC12 and SH-SY5Y cells. Parkinsonism Relat Disord 15:220–225
Um JW, Im E, Lee HJ, Min B, Yoo L, Yoo J, Lubbert H, Stichel-Gunkel C et al (2010) Parkin directly modulates 26S proteasome activity. J Neurosci 30:11805–11814. doi:10.1523/JNEUROSCI.2862-09.2010
Wang C, Ko HS, Thomas B, Tsang F, Chew KC, Tay SP, Ho MW, Lim TM et al (2005) Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin’s protective function. Hum Mol Genet 14:3885–3897
Higashi Y, Asanuma M, Miyazaki I, Hattori N, Mizuno Y, Ogawa N (2004) Parkin attenuates manganese-induced dopaminergic cell death. J Neurochem 89(6):1490–1497
Srinivasula SM, Ashwell JD (2008) IAPs: what’s in a name? Mol Cell 30:123–135. doi:10.1016/j.molcel.2008.03.008
Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC (1998) A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem 273:7787–7790
Galban S, Duckett CS (2010) XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ 17(1):54–60. doi:10.1038/cdd.2009.81
Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y (2001) Structural basis of caspase-7 inhibition by XIAP. Cell 104(5):769–780
Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS (2001) Structural basis for the inhibition of caspase-3 by XIAP. Cell 104(5):791–800
Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R et al (2003) Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell 11:519–527
Paulsen M, Ussat S, Jakob M, Scherer G, Lepenies I, Schutze S, Kabelitz D, Adam-Klages S (2008) Interaction with XIAP prevents full caspase-3/-7 activation in proliferating human T lymphocytes. Eur J Immunol 38:1979–1987. doi:10.1002/eji.200838211
Bornstein B, Gottfried Y, Edison N, Shekhtman A, Lev T, Glaser F, Larisch S (2011) ARTS binds to a distinct domain in XIAP-BIR3 and promotes apoptosis by a mechanism that is different from other IAP-antagonists. Apoptosis 16:869–881. doi:10.1007/s10495-011-0622-0
Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S (2004) The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J 23:1627–1635. doi:10.1038/sj.emboj.7600155
Kemeny S, Dery D, Loboda Y, Rovner M, Lev T, Zuri D, Finberg JP, Larisch S (2012) Parkin promotes degradation of the mitochondrial pro-apoptotic ARTS protein. PLoS One 7:e38837. doi:10.1371/journal.pone.0038837
Hu Y, Zhang N, Fan Q, Lin M, Zhang C, Fan G, Zhai X, Zhang F et al (2015) Protective efficacy of carnosic acid against hydrogen peroxide induced oxidative injury in HepG2 cells through the SIRT1 pathway. Can J Physiol Pharmacol 93:625–631. doi:10.1139/cjpp-2014-0513
Park JA, Kim S, Lee SY, Kim CS, Kim DK, Kim SJ, Chun HS (2008) Beneficial effects of carnosic acid on dieldrin-induced dopaminergic neuronal cell death. Neuroreport 19:1301–1304. doi:10.1097/WNR.0b013e32830abc1f
Tsai CW, Liu KL, Lin YR, Kuo WC (2014) The mechanisms of carnosic acid attenuates tumor necrosis factor-alpha-mediated inflammation and insulin resistance in 3 T3-L1 adipocytes. Mol Nutr Food Res 58:654–664. doi:10.1002/mnfr.201300356
Zhang D, Lee B, Nutter A, Song P, Dolatabadi N, Parker J, Sanz-Blasco S, Newmeyer T et al (2015) Protection from cyanide-induced brain injury by the Nrf2 transcriptional activator carnosic acid. J Neurochem 133:898–908. doi:10.1111/jnc.13074
Barni MV, Carlini MJ, Cafferata EG, Puricelli L, Moreno S (2012) Carnosic acid inhibits the proliferation and migration capacity of human colorectal cancer cells. Oncol Rep 27:1041–1048. doi:10.3892/or.2012.1630
Chen JH, Ou HP, Lin CY, Lin FJ, Wu CR, Chang SW, Tsai CW (2012) Carnosic acid prevents 6-hydroxydopamine-induced cell death in SH-SY5Y cells via mediation of glutathione synthesis. Chem Res Toxicol 25:1893–1901. doi:10.1021/tx300171u
Wu CR, Tsai CW, Chang SW, Lin CY, Huang LC, Tsai CW (2015) Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: involvement of antioxidative enzymes induction. Chem Biol Interact 225:40–46. doi:10.1016/j.cbi.2014.11.011
Lin CY, Chen JH, Fu RH, Tsai CW (2014) Induction of Pi form of glutathione S-transferase by carnosic acid is mediated through PI3K/Akt/NF-kappaB pathway and protects against neurotoxicity. Chem Res Toxicol 27:1958–1966. doi:10.1021/tx5003063
Lin CY, Tsai CW (2016) Carnosic acid attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells by inducing autophagy through an enhanced interaction of parkin and Beclin1. Mol Neurobiol. doi:10.1007/s12035-016-9873-7
Wilhelmus MM, Nijland PG, Drukarch B, de Vries HE, van Horssen J (2012) Involvement and interplay of parkin, PINK1, and DJ1 in neurodegenerative and neuroinflammatory disorders. Free Radic Biol Med 53:983–992. doi:10.1016/j.freeradbiomed.2012.05.040
Ono K, Ikeda T, Takasaki J, Yamada M (2011) Familial Parkinson disease mutations influence alpha-synuclein assembly. Neurobiol Dis 43:715–724. doi:10.1016/j.nbd.2011.05.025
Iaccarino C, Crosio C, Vitale C, Sanna G, Carri MT, Barone P (2007) Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum Mol Genet 16:1319–1326
Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X (2012) Parkinson's disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem 121:830–839. doi:10.1111/j.1471-4159.2012.07734.x
Qu W, Fan L, Kim YC, Ishikawa S, Iguchi-Ariga SM, Pu XP, Ariga H (2009) Kaempferol derivatives prevent oxidative stress-induced cell death in a DJ-1-dependent manner. J Pharmacol Sci 110:191–200
Ortiz-Ortiz MA, Moran JM, Ruiz-Mesa LM, Niso-Santano M, Bravo-SanPedro JM, Gomez-Sanchez R, Gonzalez-Polo RA, Fuentes JM (2010) Curcumin exposure induces expression of the Parkinson’s disease-associated leucine-rich repeat kinase 2 (LRRK2) in rat mesencephalic cells. Neurosci Lett 468:120–124. doi:10.1016/j.neulet.2009.10.081
Romani-Aumedes J, Canal M, Martin-Flores N, Sun X, Perez-Fernandez V, Wewering S, Fernandez-Santiago R, Ezquerra M et al (2014) Parkin loss of function contributes to RTP801 elevation and neurodegeneration in Parkinson’s disease. Cell Death Dis 5:e1364. doi:10.1038/cddis.2014.333
Scarffe LA, Stevens DA, Dawson VL, Dawson TM (2014) Parkin and PINK1: much more than mitophagy. Trends Neurosci 37:315–324. doi:10.1016/j.tins.2014.03.004
Dai H, Deng Y, Zhang J, Han H, Zhao M, Li Y, Zhang C, Tian J et al (2015) PINK1/parkin-mediated mitophagy alleviates chlorpyrifos-induced apoptosis in SH-SY5Y cells. Toxicology 334:72–80. doi:10.1016/j.tox.2015.06.003
Henn IH, Bouman L, Schlehe JS, Schlierf A, Schramm JE, Wegener E, Nakaso K, Culmsee C et al (2007) Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J Neurosci 27:1868–1878
Um JW, Park HJ, Song J, Jeon I, Lee G, Lee PH, Chung KC (2010) Formation of parkin aggregates and enhanced PINK1 accumulation during the pathogenesis of Parkinson’s disease. Biochem Biophys Res Commun 393:824–828. doi:10.1016/j.bbrc.2010.02.090
Obexer P, Ausserlechner MJ (2014) X-linked inhibitor of apoptosis protein—a critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol 4:197. doi:10.3389/fonc.2014.00197
Eckelman BP, Salvesen GS (2006) The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem 281:3254–3260. doi:10.1074/jbc.M510863200
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5(4):a008656. doi:10.1101/cshperspect.a008656
Tsang AH, Lee YI, Ko HS, Savitt JM, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM et al (2009) S-nitrosylation of XIAP compromises neuronal survival in Parkinson’s disease. Proc Natl Acad Sci U S A 106:4900–4905
Saito A, Hayashi T, Okuno S, Ferrand-Drake M, Chan PH (2003) Interaction between XIAP and Smac/DIABLO in the mouse brain after transient focal cerebral ischemia. J Cereb Blood Flow Metab 23:1010–1019. doi:10.1097/01.WCB.0000080702.47016.FF
Huang Y, Rich RL, Myszka DG, Wu H (2003) Requirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J Biol Chem 278:49517–49522. doi:10.1074/jbc.M310061200
Acknowledgements
This work was supported by the Ministry of Science and Technology (MOST 103-2320-B-039-042-MY3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, RH., Huang, LC., Lin, CY. et al. Modulation of ARTS and XIAP by Parkin Is Associated with Carnosic Acid Protects SH-SY5Y Cells against 6-Hydroxydopamine-Induced Apoptosis. Mol Neurobiol 55, 1786–1794 (2018). https://doi.org/10.1007/s12035-017-0443-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0443-4