Abstract
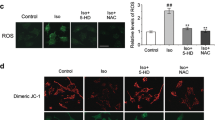
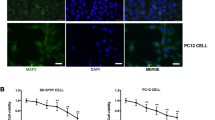
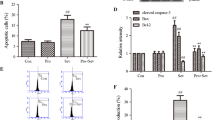
DNA damage is associated with aging and neurological disorders, including Alzheimer’s disease. Isoflurane is a commonly used anesthetic. It remains largely unknown whether isoflurane induces DNA damage. Phosphorylation of the histone protein H2A variant X at Ser139 (γH2A.X) is a marker of DNA damage. We therefore set out to assess the effects of isoflurane on γH2A.X level in H4 human neuroglioma cells and in brain tissues of mice. Oxidative stress, caspase-activated DNase (CAD), and the p53 signaling pathway are involved in DNA damage. Thus, we determined the interaction of isoflurane with reactive oxygen species (ROS), CAD, and p53 to illustrate the underlying mechanisms. The cells were treated with 2 % isoflurane for 3 or 6 h. The mice were anesthetized with 1.4 % isoflurane for 2 h. Western blot, immunostaining and live cell fluorescence staining were used in the experiments. We showed that isoflurane increased levels of γH2A.X, cleaved caspase-3, and nucleus translocation of CAD and decreased levels of inhibitor of CAD (ICAD) and p53. Isoflurane enhanced the nucleus level of γH2A.X. Moreover, caspase inhibitor Z-VAD and ROS generation inhibitor N-acetyl-L-cysteine (NAC) attenuated the isoflurane-induced increase in γH2A.X level. However, NAC did not significantly alter the isoflurane-induced reduction in p53 level. Finally, p53 activator (actinomycin D) and inhibitor (pifithrin-α) attenuated and potentiated the isoflurane-induced increase in γH2A.X level, respectively. These findings suggest that isoflurane might induce DNA damage, as represented by increased γH2A.X level, via induction of oxidative stress and inhibition of the repair of DNA damage through the p53 signaling pathway.








Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
β-amyloid protein
- DSBs:
-
DNA double-strand breaks
- ATM:
-
Ataxia telangiectasia mutated
- γH2A.X:
-
Phosphorylation of histone protein H2A variant X at Ser139
- ROS:
-
Reactive oxygen species
- CAD:
-
Caspase-activated DNase
- ICAD:
-
Inhibitor of caspase-activated DNase
- NAC:
-
N-acetyl-L-cysteine
- DCFH-DA:
-
2′,7′-Dichlorfluorescein-diacetate
References
Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM et al (2004) Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology 101(3):703–709
Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG (2005) Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Research 1037(1-2):139–147
Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, Hoetzel A, Schmidt R et al (2005) Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology 102(6):1147–1157
Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE (2006) The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 104(5):988–994
Wei H, Liang G, Yang H (2007) Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neuroscience letters 425(1):59–62
Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE (2007) The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. The Journal of Neuroscience 27(6):1247–1254
Liang G, Wang Q, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei H (2008) A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesthesia and analgesia 106(2):492–500, table of contents
Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G et al (2008) The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Annals of neurology 64(6):618–627. doi:10.1002/ana.21548
Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF (2008) Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiology of aging 29(7):1002–1010. doi:10.1016/j.neurobiolaging.2007.02.009
Valentim AM, Di Giminiani P, Ribeiro PO, Rodrigues P, Olsson IA, Antunes LM (2010) Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology 113(5):1099–1108. doi:10.1097/ALN.0b013e3181f79c7c
Lin D, Zuo Z (2011) Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology 61(8):1354–1359. doi:10.1016/j.neuropharm.2011.08.011
Culley DJ, Baxter M, Yukhananov R, Crosby G (2003) The memory effects of general anesthesia persist for weeks in young and aged rats. Anesthesia and analgesia 96(4):1004–1009, table of contents
Eger EI, Xing Y, Pearce R, Shafer S, Laster MJ, Zhang Y, Fanselow MS, Sonner JM (2003) Isoflurane antagonizes the capacity of flurothyl or 1,2-dichlorohexafluorocyclobutane to impair fear conditioning to context and tone. Anesthesia and analgesia 96(4):1010–1018, table of contents
Culley DJ, Baxter MG, Yukhananov R, Crosby G (2004) Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology 100(2):309–314
Saab BJ, Maclean AJ, Kanisek M, Zurek AA, Martin LJ, Roder JC, Orser BA (2010) Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology 113(5):1061–1071. doi:10.1097/ALN.0b013e3181f56228
Callaway JK, Jones NC, Royse CF (2012) Isoflurane induces cognitive deficits in the Morris water maze task in rats. Eur J Anaesthesiol 29(5):239–245. doi:10.1097/EJA.0b013e32835103c1
Lin D, Cao L, Wang Z, Li J, Washington JM, Zuo Z (2012) Lidocaine attenuates cognitive impairment after isoflurane anesthesia in old rats. Behavioural brain research 228(2):319–327. doi:10.1016/j.bbr.2011.12.010
Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER et al (2012) Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Annals of neurology 71(5):687–698. doi:10.1002/ana.23536
Cheng B, Zhang Y, Wang A, Dong Y, Xie Z (2015) Vitamin C attenuates isoflurane-induced caspase-3 activation and cognitive impairment. Molecular neurobiology 52(3):1580–1589. doi:10.1007/s12035-014-8959-3
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. doi:10.1016/j.cell.2013.05.039
Hughes KA, Reynolds RM (2005) Evolutionary and mechanistic theories of aging. Annual review of entomology 50:421–445. doi:10.1146/annurev.ento.50.071803.130409
Martin GM (2007) Modalities of gene action predicted by the classical evolutionary biological theory of aging. Annals of the New York Academy of Sciences 1100:14–20. doi:10.1196/annals.1395.002
Hoeijmakers JH (2009) DNA damage, aging, and cancer. The New England journal of medicine 361(15):1475–1485. doi:10.1056/NEJMra0804615
Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429(6994):883–891. doi:10.1038/nature02661
McKinnon PJ (2009) DNA repair deficiency and neurological disease. Nature reviews Neuroscience 10(2):100–112. doi:10.1038/nrn2559
Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G (2008) Nucleic acid oxidation in Alzheimer disease. Free radical biology & medicine 44(8):1493–1505. doi:10.1016/j.freeradbiomed.2008.01.002
Canugovi C, Yoon JS, Feldman NH, Croteau DL, Mattson MP, Bohr VA (2012) Endonuclease VIII-like 1 (NEIL1) promotes short-term spatial memory retention and protects from ischemic stroke-induced brain dysfunction and death in mice. Proceedings of the National Academy of Sciences of the United States of America 109(37):14948–14953. doi:10.1073/pnas.1204156109
Shiloh Y (2006) The ATM-mediated DNA-damage response: taking shape. Trends in biochemical sciences 31(7):402–410. doi:10.1016/j.tibs.2006.05.004
Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y (2008) GammaH2AX and cancer. Nature reviews Cancer 8(12):957–967. doi:10.1038/nrc2523
Garcia-Canton C, Anadon A, Meredith C (2012) gammaH2AX as a novel endpoint to detect DNA damage: applications for the assessment of the in vitro genotoxicity of cigarette smoke. Toxicol In Vitro 26(7):1075–1086. doi:10.1016/j.tiv.2012.06.006
Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S (2013) gammaH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat Res 753(1):24–40. doi:10.1016/j.mrrev.2013.02.001
Sanchez-Flores M, Pasaro E, Bonassi S, Laffon B, Valdiglesias V (2015) gammaH2AX assay as DNA damage biomarker for human population studies: defining experimental conditions. Toxicol Sci 144(2):406–413. doi:10.1093/toxsci/kfv011
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408(6809):239–247. doi:10.1038/35041687
Olovnikov IA, Kravchenko JE, Chumakov PM (2009) Homeostatic functions of the p53 tumor suppressor: regulation of energy metabolism and antioxidant defense. Seminars in cancer biology 19(1):32–41. doi:10.1016/j.semcancer.2008.11.005
Yuste VJ, Sanchez-Lopez I, Sole C, Moubarak RS, Bayascas JR, Dolcet X, Encinas M, Susin SA et al (2005) The contribution of apoptosis-inducing factor, caspase-activated DNase, and inhibitor of caspase-activated DNase to the nuclear phenotype and DNA degradation during apoptosis. The Journal of biological chemistry 280(42):35670–35683. doi:10.1074/jbc.M504015200
Sakahira H, Enari M, Nagata S (1998) Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391(6662):96–99. doi:10.1038/34214
Orth JD, Loewer A, Lahav G, Mitchison TJ (2012) Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Molecular biology of the cell 23(4):567–576. doi:10.1091/mbc.E11-09-0781
Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T et al (2010) The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. The Journal of biological chemistry 285(6):4025–4037. doi:10.1074/jbc.M109.065664
Zhang B, Dong Y, Zhang G, Moir RD, Xia W, Yue Y, Tian M, Culley DJ et al (2008) The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein levels under hypoxic conditions. The Journal of biological chemistry 283(18):11866–11875. doi:10.1074/jbc.M800199200
Chen CS, Ho DR, Chen FY, Chen CR, Ke YD, Su JG (2014) AKT mediates actinomycin D-induced p53 expression. Oncotarget 5(3):693–703. doi:10.18632/oncotarget.1328
Abdelalim EM, Tooyama I (2012) The p53 inhibitor, pifithrin-alpha, suppresses self-renewal of embryonic stem cells. Biochemical and biophysical research communications 420(3):605–610. doi:10.1016/j.bbrc.2012.03.041
Zhang Y, Shao H, Dong Y, Swain CA, Yu B, Xia W, Xie Z (2014) Chronic treatment with anesthetic propofol attenuates beta-amyloid protein levels in brain tissues of aged mice. Translational neurodegeneration 3(1):8. doi:10.1186/2047-9158-3-8
Curtin JF, Donovan M, Cotter TG (2002) Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods 265(1-2):49–72
Halasi M, Wang M, Chavan TS, Gaponenko V, Hay N, Gartel AL (2013) ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. The Biochemical journal 454(2):201–208. doi:10.1042/BJ20130282
Green DR, Kroemer G (2009) Cytoplasmic functions of the tumour suppressor p53. Nature 458(7242):1127–1130. doi:10.1038/nature07986
McKinnon PJ, Caldecott KW (2007) DNA strand break repair and human genetic disease. Annu Rev Genomics Hum Genet 8:37–55. doi:10.1146/annurev.genom.7.080505.115648
Jeggo PA, Lobrich M (2007) DNA double-strand breaks: their cellular and clinical impact? Oncogene 26(56):7717–7719. doi:10.1038/sj.onc.1210868
Hakem R (2008) DNA-damage repair; the good, the bad, and the ugly. The EMBO journal 27(4):589–605. doi:10.1038/emboj.2008.15
Rogakou EP, Boon C, Redon C, Bonner WM (1999) Megabase chromatin domains involved in DNA double-strand breaks in vivo. The Journal of cell biology 146(5):905–916
Fernandez-Capetillo O, Mahadevaiah SK, Celeste A, Romanienko PJ, Camerini-Otero RD, Bonner WM, Manova K, Burgoyne P et al (2003) H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell 4(4):497–508
Lee SY, Lau AT, Jeong CH, Shim JH, Kim HG, Kim J, Bode AM, Dong Z (2010) Histone XH2AX is required for Xenopus anterior neural development: critical role of threonine 16 phosphorylation. The Journal of biological chemistry 285(38):29525–29534. doi:10.1074/jbc.M110.127233
Fernando RN, Eleuteri B, Abdelhady S, Nussenzweig A, Andang M, Ernfors P (2011) Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proceedings of the National Academy of Sciences of the United States of America 108(14):5837–5842. doi:10.1073/pnas.1014993108
Crowe SL, Movsesyan VA, Jorgensen TJ, Kondratyev A (2006) Rapid phosphorylation of histone H2A.X following ionotropic glutamate receptor activation. The European journal of neuroscience 23(9):2351–2361. doi:10.1111/j.1460-9568.2006.04768.x
Crowe SL, Tsukerman S, Gale K, Jorgensen TJ, Kondratyev AD (2011) Phosphorylation of histone H2A.X as an early marker of neuronal endangerment following seizures in the adult rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 31(21):7648–7656. doi:10.1523/JNEUROSCI.0092-11.2011
Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT (2010) ATM activation by oxidative stress. Science 330(6003):517–521. doi:10.1126/science.1192912
Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA et al (2014) The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157(3):565–579. doi:10.1016/j.cell.2014.03.032
Fritsche M, Haessler C, Brandner G (1993) Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene 8(2):307–318
Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M et al (2009) A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 460(7259):1149–1153. doi:10.1038/nature08287
Sperka T, Wang J, Rudolph KL (2012) DNA damage checkpoints in stem cells, ageing and cancer. Nature reviews Molecular cell biology 13(9):579–590. doi:10.1038/nrm3420
Sahin E, DePinho RA (2012) Axis of ageing: telomeres, p53 and mitochondria. Nature reviews Molecular cell biology 13(6):397–404. doi:10.1038/nrm3352
Smith ML, Ford JM, Hollander MC, Bortnick RA, Amundson SA, Seo YR, Deng CX, Hanawalt PC et al (2000) p53-mediated DNA repair responses to UV radiation: studies of mouse cells lacking p53, p21, and/or gadd45 genes. Mol Cell Biol 20(10):3705–3714
Gao Y, Ferguson DO, Xie W, Manis JP, Sekiguchi J, Frank KM, Chaudhuri J, Horner J et al (2000) Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature 404(6780):897–900. doi:10.1038/35009138
Lakin ND, Jackson SP (1999) Regulation of p53 in response to DNA damage. Oncogene 18(53):7644–7655. doi:10.1038/sj.onc.1203015
Brozovic G, Orsolic N, Knezevic F, Horvat Knezevic A, Benkovic V, Sakic K, Borojevic N, Dikic D (2011) The in vivo genotoxicity of cisplatin, isoflurane and halothane evaluated by alkaline comet assay in Swiss albino mice. J Appl Genet 52(3):355–361. doi:10.1007/s13353-011-0046-0
Szyfter K, Stachecki I, Kostrzewska-Poczekaj M, Szaumkessel M, Szyfter-Harris J, Sobczynski P (2015) Exposure to volatile anaesthetics is not followed by a massive induction of single-strand DNA breaks in operation theatre personnel. J Appl Genet. doi:10.1007/s13353-015-0329-y
Leffa DD, Bristot BN, Damiani AP, Borges GD, Daumann F, Zambon GM, Fagundes GE, de Andrade VM (2015) Anesthetic ketamine-induced DNA damage in different cell types in vivo. Molecular neurobiology. doi:10.1007/s12035-015-9476-8
Santiard-Baron D, Lacoste A, Ellouk-Achard S, Soulie C, Nicole A, Sarasin A, Ceballos-Picot I (2001) The amyloid peptide induces early genotoxic damage in human preneuron NT2. Mutat Res 479(1-2):113–120
Coppede F, Migliore L (2015) DNA damage in neurodegenerative diseases. Mutat Res 776:84–97. doi:10.1016/j.mrfmmm.2014.11.010
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America 92(20):9363–9367
Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J (2003) Aging and genome maintenance: lessons from the mouse? Science 299(5611):1355–1359. doi:10.1126/science.1079161
Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW (2005) DNA repair, genome stability, and aging. Cell 120(4):497–512. doi:10.1016/j.cell.2005.01.028
Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM (2006) Cellular senescence in aging primates. Science 311(5765):1257. doi:10.1126/science.1122446
Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL (2007) Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447(7145):725–729. doi:10.1038/nature05862
Jiang H, Ju Z, Rudolph KL (2007) Telomere shortening and ageing. Z Gerontol Geriatr 40(5):314–324. doi:10.1007/s00391-007-0480-0
Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, Rube C (2011) Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PloS one 6(3):e17487. doi:10.1371/journal.pone.0017487
Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S et al (2002) Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 16(9):935–942. doi:10.1096/fj.01-0977com
O’Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA et al (2002) Chromosomal instability in ulcerative colitis is related to telomere shortening. Nature genetics 32(2):280–284. doi:10.1038/ng989
Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA (2008) An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 372(9633):139–144. doi:10.1016/S0140-6736(08)60878-8
Acknowledgments
This research was supported by R21 AG038994, R01 GM088801, and R01 AG041274 from National Institutes of Health, Bethesda, Maryland, and Investigator-initiated Research grant from Alzheimer’s Association, Chicago, Illinois to Zhongcong Xie. Anesthetic isoflurane was generously provided by the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA. The experiments were performed in Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal protocol was approved by the Massachusetts General Hospital (Boston, Massachusetts) Standing Committee on the Use of Animals in Research and Teaching. All experiments followed the National Institutes of Health guidelines, and efforts were made to minimize the number of animals used.
Conflict of Interest
The authors declare that they have no competing interests.
Author Contributions
C.N., C.L., and Y.D. performed experiments, generated and analyzed the data, contributed to project design and manuscript writing. Y.Z. and X.G. contributed to the design of experiments and data analysis. Z.X. designed and directed the project, participated in experiments, and wrote the manuscript. All authors read and approved the manuscript.
Rights and permissions
About this article
Cite this article
Ni, C., Li, C., Dong, Y. et al. Anesthetic Isoflurane Induces DNA Damage Through Oxidative Stress and p53 Pathway. Mol Neurobiol 54, 3591–3605 (2017). https://doi.org/10.1007/s12035-016-9937-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9937-8