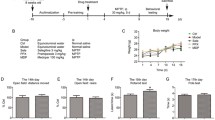
Abstract
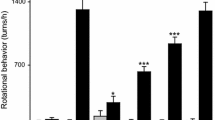
A crucial event in the pathogenesis of Parkinson’s disease is the death of dopaminergic neurons of the nigrostriatal system, which are responsible for the regulation of motor function. Motor symptoms first appear in patients 20–30 years after the onset of the neurodegeneration, when there has been a loss of an essential number of neurons and depletion of compensatory reserves of the brain, which explains the low efficiency of treatment. Therefore, the development of a technology for the diagnosing of Parkinson’s disease at the preclinical stage is of a high priority in neurology. In this study, we have developed at an experimental model a fundamentally novel for neurology approach for diagnosis of Parkinson’s disease at the preclinical stage. This methodology, widely used for the diagnosis of chronic diseases in the internal medicine, is based on the application of a challenge test that temporarily increases the latent failure of a specific functional system, thereby inducing the short-term appearance of clinical symptoms. The provocation test was developed by a systemic administration of α-methyl-p-tyrosine (αMpT), a reversible inhibitor of tyrosine hydroxylase to MPTP-treated mice at the presymptomatic stage of parkinsonism. For this, we first selected a minimum dose of αMpT, which caused a decrease of the dopamine level in the striatum of normal mice below the threshold at which motor dysfunctions appear. Then, we found the maximum dose of αMpT at which a loss of dopamine in the striatum of normal mice did not reach the threshold level, and motor behavior was not impaired. We showed that αMpT at this dose induced a decrease of the dopamine concentration in the striatum of MPTP-treated mice at the presymptomatic stage of parkinsonism below a threshold level that results in the impairment of motor behavior. Finally, we proved that αMpT exerts a temporal and reversible influence on the nigrostriatal dopaminergic system of MPTP-treated mice with no long-term side effects on other catecholaminergic systems. Thus, the above experimental data strongly suggest that αMpT-based challenge test might be considered as the provocation test for Parkinson’s disease diagnosis at the preclinical stage in the future clinical trials.







Similar content being viewed by others
Abbreviations
- AADC:
-
Aromatic L-amino acid decarboxylase
- DA:
-
Dopamine
- HPLC-ED:
-
High-performance liquid chromatography with electrochemical detection
- l-DOPA:
-
l-3,4-Dihydroxyphenylalanine
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NE:
-
Norepinephrine
- SN:
-
Substantia nigra
- αMpT:
-
α-methyl-p-tyrosine
- ТН:
-
Tyrosine hydroxylase
References
Becker G, Muller A, Braune S et al (2002) Early diagnosis of Parkinson’s disease. J Neurol 249 Suppl:III/40–III/48. doi:10.1007/s00415-002-1309-9
Spiegel J, Storch A, Jost WH (2006) Early diagnosis of Parkinson’s disease. J Neurol 253:iv2–iv7. doi:10.1007/s00415-006-4002-6
Gaenslen A, Berg D (2010) Early diagnosis of Parkinson’s disease. Int Rev Neurobiol 90:81–92. doi:10.1016/S0074-7742(10)90006-8
De Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535. doi:10.1016/S1474-4422(06)70471-9
Dorsey ER, Constantinescu R, Thompson JP et al (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68:384–386. doi:10.1212/01.wnl.0000247740.47667.03
Alves G, Forsaa EB, Pedersen KF et al (2008) Epidemiology of Parkinson’s disease. J Neurol 255 Suppl:18–32. doi:10.1007/s00415-008-5004-3
Von Campenhausen S, Winter Y, Rodrigues e Silva A (2011) Costs of illness and care in Parkinson’s disease: an evaluation in six countries. Eur Neuropsychopharmacol 21:180–191. doi:10.1016/j.euroneuro.2010.08.002
Thies W, Bleiler L, Association A, Alzheimer’s A (2013) Alzheimer’s disease facts and figures. Alzheimer’s Dement 9:208–245. doi:10.1016/j.jalz.2013.02.003
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Agid Y (1991) Parkinson’s disease: pathophysiology. Lancet 337:1321–1324
Ehringer H, Hornykiewicz O (1998) Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Park Relat Disord 4:53–57
Ugrumov MV (2008) Brain neurons partly expressing monoaminergic phenotype: distribution, development, and functional significance in norm and pathology. In: Lajtha A, Vizi ES (eds) Handb. Neurochem. Mol. Neurobiol. Springer, US, pp 21–73
Musiek ES, Schindler SE (2013) Alzheimer disease: current concepts & future directions. Mo Med 110:395–400
Willis AW (2013) Parkinson disease in the elderly adult. Mo Med 110:406–410
Bogdanov M, Matson WR, Wang L et al (2008) Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain 131:389–396. doi:10.1093/brain/awm304
Wu Y, Le W, Jankovic J et al (2011) Preclinical biomarkers of Parkinson disease. Arch Neurol 68:22–30. doi:10.1001/archneurol.2010.321
Parnetti L, Castrioto A, Chiasserini D et al (2013) Cerebrospinal fluid biomarkers in Parkinson disease. Nat Rev Neurol 9:131–140. doi:10.1038/nrneurol.2013.10
Sharma S, Moon CS, Khogali A et al (2013) Biomarkers in Parkinson’s disease (recent update). Neurochem Int 63:201–229. doi:10.1016/j.neuint.2013.06.005
Tokuda T, Qureshi MM, Ardah MT et al (2010) Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75:1766–1772. doi:10.1212/WNL.0b013e3181fd613b
DeKosky ST, Marek K (2003) Looking backward to move forward: early detection of neurodegenerative disorders. Science 302:830–834. doi:10.1126/science.1090349
Pellicano C, Benincasa D, Pisani V et al (2007) Prodromal non-motor symptoms of Parkinson’s disease. Neuropsychiatr Dis Treat 3:145–151. doi:10.2147/nedt.2007.3.1.145
Takahashi K (2013) Non-motor symptoms in premotor phase of Parkinson disease. Rinsho Shinkeigaku 53:974–976
Berg D (2006) Marker for a preclinical diagnosis of Parkinson’s disease as a basis for neuroprotection. J Neural Transm Suppl 71:123–32
Freeman RK (1975) The use of the oxytocin challenge test for antepartum clinical evaluation of uteroplacental respiratory function. Am J Obs Gynecol 121:481–489
Gambrell RD, Massey FM, Castaneda TA et al (1980) Use of the progestogen challenge test to reduce the risk of endometrial cancer. Obstet Gynecol 55:732–738
Ginsburg R, Bristow MR, Kantrowitz N et al (1981) Histamine provocation of clinical coronary artery spasm: implications concerning pathogenesis of variant angina pectoris. Am Hear J 102:819–822
Lebecque P, Spier S, Lapierre JG et al (1987) Histamine challenge test in children using forced oscillation to measure total respiratory resistance. Chest 92:313–318
Phillips LS, Ziemer DC, Kolm P et al (2009) Glucose challenge test screening for prediabetes and undiagnosed diabetes. Diabetologia 52:1798–1807. doi:10.1007/s00125-009-1407-7
Witek P, Zgliczyński W, Zieliński G, Jeske W (2010) The role of combined low-dose dexamethasone suppression test and desmopressin stimulation test in the diagnosis of persistent Cushing’s disease. Case report. Endokrynol Pol 61:312–317
Gasco V, Beccuti G, Baldini C et al (2013) Acylated ghrelin as a provocative test for the diagnosis of GH deficiency in adults. Eur J Endocrinol 168:23–30. doi:10.1530/EJE-12-0584
Hermann LK, Newman DH, Pleasant WA et al (2013) Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern Med 173:1128–1133. doi:10.1001/jamainternmed.2013.850
Porsbjerg C, Sverrild A, Backer V (2015) Combining the mannitol test and FeNO in the assessment of poorly controlled asthma. J Allergy Clin Immunol Pract 3:1–7. doi:10.1016/j.jaip.2015.02.005
Rhee N, Oh KY, Yang EM, Kim CJ (2015) Growth hormone responses to provocative tests in children with short stature. Chonnam Med J 51:33–38. doi:10.4068/cmj.2015.51.1.33
Sun Q, Sha W, Gui X-W et al (2015) Drug-induced lymphocyte stimulation test in the prediction of drug-induced hypersensitivity to antituberculosis drugs. Diagn Microbiol Infect Dis 82:172–176. doi:10.1016/j.diagmicrobio.2015.03.008
Sedelis M, Schwarting RKW, Huston JP (2001) Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav Brain Res 125:109–125. doi:10.1016/S0166-4328(01)00309-6
Rech RH, Borys HK, Moore KE (1966) Alterations in behavior and brain catecholamine levels in rats treated with alpha-methyltyrosine. J Pharmacol Exp Ther 153:412–419
Hutchins DA, Rogers KJ (1973) Effect of depletion of cerebral monoamines on the concentration of glycogen and on amphetamine-induced glycogenolysis in the brain. Br J Pharmacol 48:19–29
Relationships D (1978) Inhibition of the in vivo biosynthesis and changes of catecholamine levels in rat brain after α-methyl-p-tyrosine; time- and dose-response relationships. Naunyn-Schmiedeberg’s Arch Pharmacol 123:111–123
Paxinos G, Franklin K (2012) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates, 4th edn. Elsevier/Academic Press, Amsterdam
Ugrumov MV, Khaindrava VG, Kozina EA et al (2011) Modeling of presymptomatic and symptomatic stages of parkinsonism in mice. Neuroscience 181:175–188. doi:10.1016/j.neuroscience.2011.03.007
Carlsson A, Lindqvist M (1973) In-vivo measurements of tryptophan and tyrosine hydroxylase activities in mouse brain. J Neural Transm 34:79–91
Izvolskaia M, Duittoz AH, Tillet Y, Ugrumov MV (2009) The influence of catecholamine on the migration of gonadotropin-releasing hormone-producing neurons in the rat foetuses. Brain Struct Funct 213:289–300. doi:10.1007/s00429-008-0197-x
Ugrumov MV (2009) Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance. J Chem Neuroanat 38:241–256. doi:10.1016/j.jchemneu.2009.08.004
Arluison M, Dietl M, Thibault J (1984) Ultrastructural morphology of dopaminergic nerve terminals and synapses in the striatum of the rat using tyrosine hydroxylase immunocytochemistry: a topographical study. Brain Res Bull 13:269–285
Ugrumov MV (2013) Brain neurons partly expressing dopaminergic phenotype. Adv Pharmacol 68:37–91. doi:10.1016/B978-0-12-411512-5.00004-X
Gobert A, Billiras R, Cistarelli L, Millan MJ (2004) Quantification and pharmacological characterization of dialysate levels of noradrenaline in the striatum of freely-moving rats: release from adrenergic terminals and modulation by alpha2-autoreceptors. J Neurosci Methods 140:141–152. doi:10.1016/j.jneumeth.2004.04.040
Kurosaki R, Muramatsu Y, Watanabe H et al (2003) Role of dopamine transporter against MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity in mice. Metab Brain Dis 18:139–146
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247
Gerfen CR, Herkenham M, Thibault J (1987) The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci 7:3915–3934
Gurevich IB, Kozina EA, Myagkov AA et al (2010) Automating extraction and analysis of dopaminergic axon terminals in images of frontal slices of the striatum. Pattern Recognit Image Anal Adv Math Theory Appl 20:349–359. doi:10.1134/S1054661810030119
Smolen AJ (1990) Image analytic techniques for quantification of immunohistochemical staining in the nervous system. Methods Neurosci 3:208–229
Borke RC, Curtis M, Ginsberg C (1993) Choline acetyltransferase and calcitonin gene-related peptide immunoreactivity in motoneurons after different types of nerve injury. J Neurocytol 22:141–153
Lucas LR, Harlan RE (1995) Cholinergic regulation of tachykinin- and enkephalin-gene expression in the rat striatum. Brain Res Mol Brain Res 30:181–195
Chang HM, Wu UI, Lan CT (2009) Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res 47:211–220. doi:10.1111/j.1600-079X.2009.00704.x
Balan IS, Ugrumov MV, Calas A et al (2000) Tyrosine hydroxylase-expressing and/or aromatic L-amino acid decarboxylase-expressing neurons in the mediobasal hypothalamus of perinatal rats: differentiation and sexual dimorphism. J Comp Neurol 425:167–176
Abramova MA, Calas A, Ugrumov MV (2011) Vasopressinergic neurons of the supraoptic nucleus in perinatal rats: reaction to osmotic stimulation and its regulation. Brain Struct Funct 215:195–207. doi:10.1007/s00429-010-0290-9
Levitt M, Spector S, Sjoerdsma A, Udenfriend S (1965) Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J Pharmacol Exp Ther 148:1–8
Bernheimer H, Birkmayer W, Hornykiewicz O et al (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S (1995) Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4:257–269
Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M et al (1999) The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol 156:50–61. doi:10.1006/exnr.1998.6993
Wu D-C, Teismann P, Tieu K et al (2003) NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A 100:6145–6150. doi:10.1073/pnas.0937239100
Luchtman DW, Shao D, Song C (2009) Behavior, neurotransmitters and inflammation in three regimens of the MPTP mouse model of Parkinson’s disease. Physiol Behav 98:130–138. doi:10.1016/j.physbeh.2009.04.021
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. doi:10.1038/nature05292
Bezard E, Gross CE, Fournier MC et al (1999) Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp Neurol 155:268–273. doi:10.1006/exnr.1998.6995
Kim RH, Smith PD, Aleyasin H et al (2005) Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A 102:5215–5220. doi:10.1073/pnas.0501282102
Dauer W, Kholodilov N, Vila M et al (2002) Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A 99:14524–14529. doi:10.1073/pnas.172514599
Fuller RW, Steranka LR (1985) Central and peripheral catecholamine depletion by 1-methyl-4-phenyl-tetrahydropyridine (MPTP) in rodents. Life Sci 36:243–247
Seniuk NA, Tatton WG, Greenwood CE (1990) Dose-dependent destruction of the coeruleus-cortical and nigral-striatal projections by MPTP. Brain Res 527:7–20
Petrucelli L, Dickson DW (2008) Neuropathology of Parkinson’s disease. In: Nass R, Przedborski S (eds) Parkinson’s disease: molecular and therapeutic insights from model systems. Elsevier Inc, Amsterdam, pp 35–48
Wu DC, Jackson-Lewis V, Vila M et al (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 22:1763–1771
Teismann P, Ferger B (2001) Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse 39:167–174. doi:10.1002/1098-2396(200102)39:2<167::AID-SYN8>3.0.CO;2-U
Du Y, Ma Z, Lin S et al (2001) Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A 98:14669–14674. doi:10.1073/pnas.251341998
German DC, Dubach M, Askari S et al (1988) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience 24:161–174
Rousselet E, Joubert C, Callebert J et al (2003) Behavioral changes are not directly related to striatal monoamine levels, number of nigral neurons, or dose of parkinsonian toxin MPTP in mice. Neurobiol Dis 14:218–228
Sandyk R, Iacono RP, Bamford CR (1987) The hypothalamus in Parkinson disease. Ital J Neurol Sci 8:227–234
Halliday GM, Blumbergs PC, Cotton RG et al (1990) Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res 510:104–107
Spector S, Sjoerdsma A, Udenfriend S (1965) Blockade of endogenous norepinephrine synthesis by agr-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J Pharmacol Exp Ther 147:86–95
Moore KE, Rech RH (1967) Antagonism by monoamine oxidase inhibitors of alpha-methyltyrosine-induced catecholamine depletion and behavioral depression. J Pharmacol Exp Ther 156:70–75
Moore KE (1968) Behavioural effects of alpha-methyltyrosine administered in the diets of mice pretreated with a monoamine oxidase inhibitor. J Pharm Pharmacol 20:656–657
Dominic JA, Moore KE (1969) Acute effects of alpha-methyltyrosine on brain catecholamine levels and on spontaneous and amphetamine-stimulated motor activity in mice. Arch Int Pharmacodyn Ther 178:166–176
Corrodi H, Hanson LC (1966) Central effects of an inhibitor of tyrosine hydroxylation. Psychopharmacologia 10:116–125
Dolphin AC, Jenner P, Marsden CD (1976) The relative importance of dopamine and noradrenaline receptor stimulation for the restoration of motor activity in reserpine or alpha-methyl-p-tyrosine pre-treated mice. Pharmacol Biochem Behav 4:661–670
Lorenc-Koci E, Ossowska K, Wardas J, Wolfarth S (1995) Does reserpine induce parkinsonian rigidity? J Neural Transm Park Dis Dement Sect 9:211–223
Bloemen OJN, De Koning MB, Boot E et al (2008) Challenge and therapeutic studies using alpha-methyl-para-tyrosine (AMPT) in neuropsychiatric disorders: a review. Cent Nerv Syst Agents Med Chem (Formerly Curr Med Chem Nerv Syst Agents) 8:249–256
Perry RR, Keiser HR, Norton JA et al (1990) Surgical management of pheochromocytoma with the use of metyrosine. Ann Surg 212:621–628
Stine SM, Krystal JH, Petrakis IL et al (1997) Effect of alpha-methyl-para-tyrosine on response to cocaine challenge. Biol Psychiatry 42:181–190. doi:10.1016/S0006-3223(96)00331-9
Turner MC, Lieberman E, DeQuattro V (1992) The perioperative management of pheochromocytoma in children. Clin Pediatr (Phila) 31:583–589. doi:10.1053/jcan.2002.124150
Engelman K, Horwitz D, Jequier E, Sjoerdsma A (1968) Biochemical and pharmacologic effects of alpha-methyltyrosine in man. J Clin Invest 47:577–594. doi:10.1172/JCI105754
Jones NF, Walker G, Ruthven CR, Sandler M (1968) Alpha-methyl-p-tyrosine in the management of phaeochromocytoma. Lancet (London, England) 2:1105–1109
Boot E, Booij J, Hasler G et al (2008) AMPT-induced monoamine depletion in humans: evaluation of two alternative [123I]IBZM SPECT procedures. Eur J Nucl Med Mol Imaging 35:1350–1356. doi:10.1007/s00259-008-0739-8
Boot E, Booij J, Zinkstok J et al (2008) Disrupted dopaminergic neurotransmission in 22q11 deletion syndrome. Neuropsychopharmacology 33:1252–1258. doi:10.1038/sj.npp.1301508
Lang AE, Marsden CD (1982) Alpha methylparatyrosine and tetrabenazine in movement disorders. Clin Neuropharmacol 5:375–387
Ankenman R, Salvatore MF (2007) Low dose alpha-methyl-para-tyrosine (AMPT) in the treatment of dystonia and dyskinesia. J Neuropsychiatry Clin Neurosci 19:65–69. doi:10.1176/jnp.2007.19.1.65
Birkmayer W, Mentasti M (1967) Further experimental studies on the catecholamine metabolism in extrapyramidal diseases (Parkinson and chorea syndromes). Arch Psychiatr Nervenkr 210:29–35
Hrachovy RA, Frost JDJ, Glaze DG, Rose D (1989) Treatment of infantile spasms with methysergide and alpha-methylparatyrosine. Epilepsia 30:607–610
Laruelle M, D’Souza CD, Baldwin RM et al (1997) Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17:162–174. doi:10.1016/S0893-133X(97)00043-2
Booij L, Van der Does AJ, Riedel WJ (2003) Monoamine depletion in psychiatric and healthy populations: review. Mol Psychiatry 8:951–973. doi:10.1038/sj.mp.4001423
Becker G, Seufert J, Bogdahn U et al (1995) Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45:182–184
Acknowledgements
We thank Ekaterina Degtyareva and Anna Kolacheva for technical assistance.
Authors’ Contributions
G.R.K. performed the motor behavior experiments, HPLC and statistical analysis; E.A.K. performed immunohistochemistry and statistical analysis; V.G.K. assisted with motor behavior experiments; G.R.K., E.A.K. analyzed the data; M.V.U. initiated and coordinated the study; G.R.K., E.A.K., M.V.U. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experimental procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 and the UK Animals (Scientific Procedures) Act 1986 and associated guidelines, or the European Communities Council Directive of 24 November 1986 (86/609/EEC) for care and use of laboratory animals and were approved by the Animal Care and Use Committee of the Institute of Developmental Biology of the Russian Academy of Sciences.
Competing Interests
The authors declare that they have no competing interests.
Funding
This work was supported by the Federal Targeted Programme Research and Development in Priority Areas of Scientific and Technological Complex of Russia for 2014–2020 years of the Ministry of Education and Science of RF (a contract No 14.604.21.0073 for 2014–2016).
Additional information
Gulnara R. Khakimova and Elena A. Kozina contributed equally to this work.
Rights and permissions
About this article
Cite this article
Khakimova, G.R., Kozina, E.A., Kucheryanu, V.G. et al. Reversible Pharmacological Induction of Motor Symptoms in MPTP-Treated Mice at the Presymptomatic Stage of Parkinsonism: Potential Use for Early Diagnosis of Parkinson’s Disease. Mol Neurobiol 54, 3618–3632 (2017). https://doi.org/10.1007/s12035-016-9936-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9936-9