Abstract
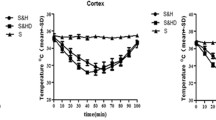
Previous studies have demonstrated depressive or hibernation-like roles of phenothiazine neuroleptics [combined chlorpromazine and promethazine (C + P)] in brain activity. This ischemic stroke study aimed to establish neuroprotection by reducing oxidative stress and improving brain metabolism with post-ischemic C + P administration. Sprague-Dawley rats were subjected to transient (2 or 4 h) middle cerebral artery occlusion (MCAO) followed by 6 or 24 h reperfusion, or permanent (28 h) MCAO without reperfusion. At 2 h after ischemia onset, rats received either an intraperitoneal (IP) injection of saline or two doses of C + P. Body temperatures, brain infarct volumes, and neurological deficits were examined. Oxidative metabolism and stress were determined by levels of ATP, NADH, and reactive oxygen species (ROS). Protein kinase C-δ (PKC-δ) and Akt expression were determined by Western blotting. C + P administration induced a neuroprotection in both transient and permanent ischemia models evidenced by significant reduction in infarct volumes and neurological deficits post-stroke. C + P induced a dose-dependent reduction in body temperature as early as 5 min post-ischemia and lasted up to 12 h. However, reduction in body temperature either only slightly or did not enhance C + P-induced neuroprotection. C + P therapy improved brain metabolism as determined by increased ATP levels and NADH activity, as well as decreased ROS production. These therapeutic effects were associated with alterations in PKC-δ and Akt protein expression. C + P treatments conferred neuroprotection in severe stroke models by suppressing the damaging cascade of metabolic events, most likely independent of drug-induced hypothermia. These findings further prove the clinical potential for C + P treatment and may direct us closer towards the development of an efficacious neuroprotective therapy.







Similar content being viewed by others
Change history
28 June 2017
An erratum to this article has been published.
References
Go AS, Mozaffarian D, Roger VL et al (2014) Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129(3):e28–e292
Kassem-Moussa H, Graffagnino C (2002) Nonocclusion and spontaneous recanalization rates in acute ischemic stroke: a review of cerebral angiography studies. Arch Neurol 59(12):1870–1873
Michalski D, Hartig W, Schneider D, Hobohm C (2011) Use of normobaric and hyperbaric oxygen in acute focal cerebral ischemia—a preclinical and clinical review. Acta Neurol Scand 123(2):85–97
Kent TA, Mandava P (2007) Recanalization rates can be misleading. Stroke 38(10):e103 author reply e104
Roth MB, Nystul T (2005) Buying time in suspended animation. Sci Am 292(6):48–55
Nathaniel TI (2008) Brain-regulated metabolic suppression during hibernation: a neuroprotective mechanism for perinatal hypoxia-ischemia. Int J Stroke 3(2):98–104
Dave KR, Christian SL, Perez-Pinzon MA, Drew KL (2012) Neuroprotection: lessons from hibernators. Comp Biochem Physiol B Biochem Mol Biol 162(1–3):1–9
Seyfried FJ, Adachi N, Arai T (2005) Suppression of energy requirement by lidocaine in the ischemic mouse brain. J Neurosurg Anesthesiol 17(2):75–81
Yenari M, Kitagawa K, Lyden P, Perez-Pinzon M (2008) Metabolic downregulation: a key to successful neuroprotection? Stroke 39(10):2910–2917
Kim J, Yenari M (2015) Hypothermia for treatment of stroke. Brain Circulation 1(1):14–25. doi:10.4103/2394-8108.164997
Lopez-Munoz F, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G (2005) History of the discovery and clinical introduction of chlorpromazine. Ann Clin Psychiatry 17(3):113–135
MacMillan V (1982) Effects of promethazine on the energy metabolism of normoxic and hypoxic rat brain. Stroke 13(4):464–469
Narayanan MV, Zhang W, Stavrovskaya IG, Kristal BS, Friedlander RM (2004) Promethazine: a novel application as a neuroprotectant that reduces ischemia-mediated injury by inhibiting mitochondrial dysfunction. Clin Neurosurg 51:102–107
Chien KR, Abrams J, Pfau RG, Farber JL (1977) Prevention by chlorpromazine of ischemic liver cell death. Am J Pathol 88(3):539–557
Liu S, Geng X, Forreider B et al (2015) Enhanced beneficial effects of mild hypothermia by phenothiazine drugs in stroke therapy. Neurol Res 37(5):454–460
Liebeskind DS (2003) Collateral circulation. Stroke 34(9):2279–2284
Liebeskind DS (2014) Collateral lessons from recent acute ischemic stroke trials. Neurol Res 36(5):397–402
Liebeskind D (2015) The collaterome: a novel conceptual framework of systems biology in cerebrovascular disorders. Brain Circulation 1(1):3–8. doi:10.4103/2394-8108.162411
Chou WH, Messing RO (2005) Protein kinase C isozymes in stroke. Trends Cardiovasc Med 15(2):47–51
Wang F, Luo Y, Ling F et al (2010) Comparison of neuroprotective effects in ischemic rats with different hypothermia procedures. Neurol Res 32(4):378–383
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91
Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD (1996) Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 27(9):1616–1622 discussion 1623
Wang F, Wang Y, Geng X et al (2012) Neuroprotective effect of acute ethanol administration in a rat with transient cerebral ischemia. Stroke 43(1):205–210
Kochanski R, Peng C, Higashida T et al (2013) Neuroprotection conferred by post-ischemia ethanol therapy in experimental stroke: an inhibitory effect on hyperglycolysis and NADPH oxidase activation. J Neurochem 126(1):113–121
Geng X, Fu P, Ji X et al (2013) Synergetic neuroprotection of normobaric oxygenation and ethanol in ischemic stroke through improved oxidative mechanism. Stroke 44(5):1418–1425
Garcia JH, Liu KF, Ho KL (1995) Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke 26(4):636–642 discussion 643
Fisher M, Takano K (1995) The penumbra, therapeutic time window and acute ischaemic stroke. Baillieres Clin Neurol 4(2):279–295
Ginsberg MD, Pulsinelli WA (1994) The ischemic penumbra, injury thresholds, and the therapeutic window for acute stroke. Ann Neurol 36(4):553–554
Cote CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C (2000) Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics 106(4):633–644
Burn JH (1954) The pharmacology of chlorpromazine and promethazine. Proc R Soc Med 47(8):617–621
Li HJ, Zhang YJ, Zhou L et al (2014) Chlorpromazine confers neuroprotection against brain ischemia by activating BKCa channel. Eur J Pharmacol 735:38–43
Zarnowska ED, Mozrzymas JW (2001) Differential effects of chlorpromazine on ionotropic glutamate receptors in cultured rat hippocampal neurons. Neurosci Lett 305(1):53–56
Sader AA, Barbieri-Neto J, Sader SL, Mazzetto SA, Alves P Jr, Vanni JC (2002) The protective action of chlorpromazine on the spinal cord of rabbits submitted to ischemia and reperfusion is dose-dependent. J Cardiovasc Surg 43(6):827–831
Stone JM, Pilowsky LS (2007) Novel targets for drugs in schizophrenia. CNS Neurol Disord Drug Targets 6(4):265–272
Wu J, Song R, Song W et al (2011) Chlorpromazine protects against apoptosis induced by exogenous stimuli in the developing rat brain. PLoS One 6(7):e21966
Bastianetto S, Danik M, Mennicken F, Williams S, Quirion R (2006) Prototypical antipsychotic drugs protect hippocampal neuronal cultures against cell death induced by growth medium deprivation. BMC Neurosci 7:28
Adolph O, Koster S, Georgieff M, Georgieff EM, Moulig W, Fohr KJ (2012) Promethazine inhibits NMDA-induced currents—new pharmacological aspects of an old drug. Neuropharmacology 63(2):280–291
Sharma A, Hamelin BA (2003) Classic histamine H1 receptor antagonists: a critical review of their metabolic and pharmacokinetic fate from a bird’s eye view. Curr Drug Metab 4(2):105–129
Morota S, Mansson R, Hansson MJ et al (2009) Evaluation of putative inhibitors of mitochondrial permeability transition for brain disorders—specificity vs. toxicity. Exp Neurol 218(2):353–362
Cleren C, Starkov AA, Calingasan NY, Lorenzo BJ, Chen J, Beal MF (2005) Promethazine protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Neurobiol Dis 20(3):701–708
Cleren C, Calingasan NY, Starkov A et al (2010) Promethazine protects against 3-nitropropionic acid-induced neurotoxicity. Neurochem Int 56(2):208–212
Gonzalez-Munoz GC, Arce MP, Lopez B et al (2010) Old phenothiazine and dibenzothiadiazepine derivatives for tomorrow’s neuroprotective therapies against neurodegenerative diseases. Eur J Med Chem 45(12):6152–6158
Forreider B, Pozivilko D, Kawaji Q, Geng X, Ding Y (2016) Hibernation-like neuroprotection in stroke by attenuating brain metabolic dysfunction. Prog Neurobiol. doi:10.1016/j.pneurobio.2016.03.002
Zager EL, Ames A 3rd (1988) Reduction of cellular energy requirements. Screening for agents that may protect against CNS ischemia. J Neurosurg 69(4):568–579
Krausz Y, Eylon L, Cerasi E (1987) Calcium-binding proteins and insulin release. Differential effects of phenothiazines on first- and second-phase secretion and on islet cAMP response to glucose. Acta Endocrinol 116(2):241–246
Larsson S (1961) The effect of chlorpromazine on the glucose metabolism in different parts of the goat brain. Acta Physiol Scand 53:68–74
Dwyer DS, Liu Y, Bradley RJ (1999) Dopamine receptor antagonists modulate glucose uptake in rat pheochromocytoma (PC12) cells. Neurosci Lett 274(3):151–154
Skinner A, Spector RG (1968) The effect of chlorpromazine on 14-C-glucose metabolism in mouse liver and brain. Br J Pharmacol Chemother 33(1):129–135
Nishibori M, Itoh Y, Oishi R, Saeki K (1987) Mechanism of the central hyperglycemic action of histamine in mice. J Pharmacol Exp Ther 241(2):582–586
Nagai K, Frohman LA (1978) Neurotensin hyperglycemia: evidence for histamine mediation and the assessment of a possible physiologic role. Diabetes 27(5):577–582
Bachelard HS, Lindsay JR (1966) Effects of neurotropic drugs on glucose metabolism in rat brain in vivo. Biochem Pharmacol 15(8):1053–1058
Bachelard HS, Gaitonde MK, Vrba R (1966) The effect of psychotropic drugs on the utilization of glucose carbon atoms in the brain, heart and liver of the rat. Biochem Pharmacol 15(8):1039–1043
Berntman L, Carlsson C (1978) Influence of “lytic cocktail” on blood flow and oxygen consumption in the rat brain. Acta Anaesthesiol Scand 22(5):515–518
Gao X, Zhang H, Takahashi T et al (2008) The Akt signaling pathway contributes to postconditioning’s protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem 105(3):943–955
Dave KR, Bhattacharya SK, Saul I et al (2011) Activation of protein kinase C delta following cerebral ischemia leads to release of cytochrome C from the mitochondria via bad pathway. PLoS One 6(7):e22057
Miele C, Paturzo F, Teperino R et al (2007) Glucose regulates diacylglycerol intracellular levels and protein kinase C activity by modulating diacylglycerol kinase subcellular localization. J Biol Chem 282(44):31835–31843
Li AJ, Oomura Y, Sasaki K, Suzuki K, Hori T (1999) Protective effect of acidic fibroblast growth factor against ischemia-induced learning and memory deficits in two tasks in gerbils. Physiol Behav 66(4):577–583
Bland ST, Tamlyn JP, Barrientos RM et al (2007) Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience 144(4):1219–1228
Reuss B, von Bohlen und Halbach O (2003) Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res 313(2):139–157
Lawlor MA, Alessi DR (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114(Pt 16):2903–2910
Daniel PM (1976) Anatomy of the hypothalamus and pituitary gland. J Clin Pathol Suppl (Assoc Clin Pathol) 7:1–7
Marinkovic SV, Milisavljevic MM, Marinkovic ZD (1989) Microanatomy and possible clinical significance of anastomoses among hypothalamic arteries. Stroke 20(10):1341–1352
DeGraba TJ, Hallenbeck JM, Pettigrew KD, Dutka AJ, Kelly BJ (1999) Progression in acute stroke: value of the initial NIH stroke scale score on patient stratification in future trials. Stroke 30(6):1208–1212
Bang OY, Saver JL, Kim SJ et al (2011) Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 42(8):2235–2239
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experimental procedures were approved by the Institutional Animal Investigation Committee of Capital Medical University in accordance with the National Institutes of Health (USA) guidelines for care and use of laboratory animals.
Sources of Funding
This work was partially supported by American Heart Association Grant-in-Aid (14GRNT20460246), Merit Review Award (I01RX-001,964-01) from the US Department of Veterans Affairs Rehabilitation R&D Service, National Natural Science Foundation of China (81501141), and Beijing NOVA program (xx2016061) as well as National Outstanding Youth Science Fund of China (no. 81325007).
Disclosures
None.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s12035-017-0656-6.
Rights and permissions
About this article
Cite this article
Geng, X., Li, F., Yip, J. et al. Neuroprotection by Chlorpromazine and Promethazine in Severe Transient and Permanent Ischemic Stroke. Mol Neurobiol 54, 8140–8150 (2017). https://doi.org/10.1007/s12035-016-0280-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0280-x