Abstract
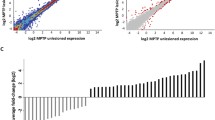
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases. Despite progress in the study of the molecular, genetic, and pathogenic mechanisms of PD, it is unclear which processes trigger the development of the pathology associated with PD. Models of the presymptomatic and early symptomatic stages of PD induced by MPTP have been used to analyze changes in transcriptome profile in brain tissues, to identify specific patterns and mechanisms underlying neurodegeneration in PD. The whole-transcriptome analysis in the brain tissues of the mice with MPTP-induced PD showed that striatum is involved in the pathogenesis in the earliest stages and the processes associated with vesicular transport may be altered. The expression profiles of the genes studied in the substantia nigra and peripheral blood confirm that lymphocytes from peripheral blood may reflect processes occurring in the brain. These data suggest that messenger RNA (mRNA) levels in peripheral blood may provide potential biomarkers of the neurodegeneration occurring in PD. The changes in expression at the mRNA and protein levels suggest that Snca may be involved in neurodegeneration and Drd2 may participate in the development of the compensatory mechanisms in the early stages of PD pathogenesis. Our data suggest that the brain cortex may be involved in the pathological processes in the early stages of PD, including the presymptomatic stage.




Similar content being viewed by others
References
Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B (2005) Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology 64(2):230–235. doi:10.1212/01.WNL.0000149511.19487.44
Claveria LE, Duarte J, Sevillano MD, Perez-Sempere A, Cabezas C, Rodriguez F, de Pedro-Cuesta J (2002) Prevalence of Parkinson’s disease in Cantalejo, Spain: a door-to-door survey. Movement Disorders : Official Journal of the Movement Disorder Society 17(2):242–249
van de Vijver DA, Roos RA, Jansen PA, Porsius AJ, de Boer A (2001) Estimation of incidence and prevalence of Parkinson’s disease in the elderly using pharmacy records. Pharmacoepidemiol Drug Saf 10(6):549–554. doi:10.1002/pds.624
Chan DK, Cordato D, Karr M, Ong B, Lei H, Liu J, Hung WT (2005) Prevalence of Parkinson’s disease in Sydney. Acta Neurol Scand 111(1):7–11. doi:10.1111/j.1600-0404.2004.00348.x
Morioka S, Sakata K, Yoshida S, Nakai E, Shiba M, Yoshimura N, Hashimoto T (2002) Incidence of Parkinson disease in Wakayama, Japan. Journal of Epidemiology / Japan Epidemiological Association 12(6):403–407
Nussbaum RL, Polymeropoulos MH (1997) Genetics of Parkinson’s disease. Hum Mol Genet 6(10):1687–1691
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Cookson MR, Hardy J, Lewis PA (2008) Genetic neuropathology of Parkinson’s disease. Int J Clin Exp Pathol 1:217–231
Fowler CJ (2007) Update on the neurology of Parkinson’s disease. Neurourol Urodyn 26(1):103–109. doi:10.1002/nau.20371
Lesage S, Brice A (2009) Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet 18(R1):R48–R59. doi:10.1093/hmg/ddp012
Coppede F (2012) Genetics and epigenetics of Parkinson’s disease. Sci World J 2012:489830. doi:10.1100/2012/489830
Lesage S, Brice A (2012) Role of mendelian genes in “sporadic” Parkinson’s disease. Parkinsonism Relat Disord 18(Suppl 1):S66–S70. doi:10.1016/S1353-8020(11)70022-0
Fox SH, Brotchie JM (2010) The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog Brain Res 184:133–157. doi:10.1016/S0079-6123(10)84007-5
Pienaar IS, Gotz J, Feany MB (2010) Parkinson’s disease: insights from non-traditional model organisms. Prog Neurobiol 92(4):558–571. doi:10.1016/j.pneurobio.2010.09.001
Byers B, Lee HL, Reijo Pera R (2012) Modeling Parkinson’s disease using induced pluripotent stem cells. Current Neurology and Neuroscience Reports 12(3):237–242. doi:10.1007/s11910-012-0270-y
German DC, Nelson EL, Liang CL, Speciale SG, Sinton CM, Sonsalla PK (1996) The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration : A Journal for Neurodegenerative Disorders, Neuroprotection, and Neuroregeneration 5(4):299–312
Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ (2008) Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord 14(Suppl 2):S112–S115. doi:10.1016/j.parkreldis.2008.04.012
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 103(8–9):987–1041
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science (New York, NY) 219(4587):979–980
Przedborski S, Jackson-Lewis V, Djaldetti R, Liberatore G, Vila M, Vukosavic S, Almer G (2000) The parkinsonian toxin MPTP: action and mechanism. Restor Neurol Neurosci 16(2):135–142
Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S (1995) Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration: A Journal for Neurodegenerative Disorders, Neuroprotection, and Neuroregeneration 4(3):257–269
Perier C, Bove J, Wu DC, Dehay B, Choi DK, Jackson-Lewis V, Rathke-Hartlieb S, Bouillet P et al (2007) Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc Natl Acad Sci U S A 104(19):8161–8166. doi:10.1073/pnas.0609874104
Prediger RD, Aguiar AS Jr, Rojas-Mayorquin AE, Figueiredo CP, Matheus FC, Ginestet L, Chevarin C, Bel ED et al (2010) Single intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson’s disease. Neurotox Res 17(2):114–129. doi:10.1007/s12640-009-9087-0
Linder JC, Klemfuss H, Groves PM (1987) Acute ultrastructural and behavioral effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice. Neurosci Lett 82(2):221–226
Ugrumov MV, Khaindrava VG, Kozina EA, Kucheryanu VG, Bocharov EV, Kryzhanovsky GN, Kudrin VS, Narkevich VB et al (2011) Modeling of presymptomatic and symptomatic stages of parkinsonism in mice. Neuroscience 181:175–188. doi:10.1016/j.neuroscience.2011.03.007
Sundstrom E, Luthman J, Jonsson G, Goldstein M (1987) Determination of monoamines by use of liquid chromatography with electrochemical detection in the study of selective monoamine neurotoxins. Life Sci 41(7):857–860
Kolacheva AA, Kozina EA, Volina EV, Ugryumov MV (2014) Time course of degeneration of dopaminergic neurons and respective compensatory processes in the nigrostriatal system in mice. Doklady Biological Sciences : Proceedings of the Academy of Sciences of the USSR, Biological Sciences Sections / Translated from Russian 456(1):160–164. doi:10.1134/s0012496614030041
Kozina EA, Khakimova GR, Khaindrava VG, Kucheryanu VG, Vorobyeva NE, Krasnov AN, Georgieva SG, Kerkerian-Le Goff L et al (2014) Tyrosine hydroxylase expression and activity in nigrostriatal dopaminergic neurons of MPTP-treated mice at the presymptomatic and symptomatic stages of parkinsonism. J Neurol Sci 340(1–2):198–207. doi:10.1016/j.jns.2014.03.028
Khakimova GR, Kozina EA, Kucheryanu VG, Ugrumov MV (2016) Reversible pharmacological induction of motor symptoms in MPTP-treated mice at the presymptomatic stage of parkinsonism: potential use for early diagnosis of Parkinson’s disease. Mol Neurobiol. doi:10.1007/s12035-016-9936-9
National Research Council (2011). Guide for the care and use of laboratory animals. (8th Edition). Washington, DC: The National Academies Press.
Suslov O, Steindler DA (2005) PCR inhibition by reverse transcriptase leads to an overestimation of amplification efficiency. Nucleic Acids Res 33(20):e181. doi:10.1093/nar/gni176
Warrington JA, Nair A, Mahadevappa M, Tsyganskaya M (2000) Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol Genomics 2(3):143–147
Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1):44–57. doi:10.1038/nprot.2008.211
Huang da W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37(1):1–13. doi:10.1093/nar/gkn923
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Antony PM, Diederich NJ, Kruger R, Balling R (2013) The hallmarks of Parkinson’s disease. FEBS J 280(23):5981–5993. doi:10.1111/febs.12335
Cheng HC, Ulane CM, Burke RE (2010) Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67(6):715–725. doi:10.1002/ana.21995
Sterling NW, Du G, Lewis MM, Dimaio C, Kong L, Eslinger PJ, Styner M, Huang X (2013) Striatal shape in Parkinson’s disease. Neurobiol Aging 34(11):2510–2516. doi:10.1016/j.neurobiolaging.2013.05.017
Gardoni F, Di Luca M (2015) Targeting glutamatergic synapses in Parkinson’s disease. Curr Opin Pharmacol 20c:24–28. doi:10.1016/j.coph.2014.10.011
Macpherson T, Morita M, Hikida T (2014) Striatal direct and indirect pathways control decision-making behavior. Front Psychol 5:1301. doi:10.3389/fpsyg.2014.01301
Hunn BH, Cragg SJ, Bolam JP, Spillantini MG, Wade-Martins R (2015) Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci 38(3):178–188. doi:10.1016/j.tins.2014.12.009
Barbanti P, Fabbrini G, Ricci A, Cerbo R, Bronzetti E, Caronti B, Calderaro C, Felici L et al (1999) Increased expression of dopamine receptors on lymphocytes in Parkinson’s disease. Movement Disorders : Official Journal of the Movement Disorder Society 14:764–771
Buttarelli FR, Capriotti G, Pellicano C, Prosperi D, Circella A, Festa A, Giovannelli M, Tofani A et al (2009) Central and peripheral dopamine transporter reduction in Parkinson’s disease. Neurol Res 31(7):687–691
Nagai Y, Ueno S, Saeki Y, Soga F, Hirano M, Yanagihara T (1996) Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson’s disease. Neurology 46:791–795
Ozansoy M, Basak AN (2013) The central theme of Parkinson’s disease: alpha-synuclein. Mol Neurobiol 47(2):460–465. doi:10.1007/s12035-012-8369-3
Noble EP (2003) D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics : the Official Publication of the International Society of Psychiatric Genetics 116b(1):103–125. doi:10.1002/ajmg.b.10005
Rieck M, Schumacher-Schuh AF, Altmann V, Francisconi CL, Fagundes PT, Monte TL, Callegari-Jacques SM, Rieder CR et al (2012) DRD2 haplotype is associated with dyskinesia induced by levodopa therapy in Parkinson’s disease patients. Pharmacogenomics 13(15):1701–1710. doi:10.2217/pgs.12.149
Kaplan N, Vituri A, Korczyn AD, Cohen OS, Inzelberg R, Yahalom G, Kozlova E, Milgrom R et al (2014) Sequence variants in SLC6A3, DRD2, and BDNF genes and time to levodopa-induced dyskinesias in Parkinson’s disease. Journal of Molecular Neuroscience : MN 53(2):183–188. doi:10.1007/s12031-014-0276-9
Goetz CG, Burke PF, Leurgans S, Berry-Kravis E, Blasucci LM, Raman R, Zhou L (2001) Genetic variation analysis in parkinson disease patients with and without hallucinations: case-control study. Arch Neurol 58(2):209–213
Kaiser R, Hofer A, Grapengiesser A, Gasser T, Kupsch A, Roots I, Brockmoller J (2003) L -dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology 60(11):1750–1755
Schumacher-Schuh AF, Rieder CR, Hutz MH (2014) Parkinson’s disease pharmacogenomics: new findings and perspectives. Pharmacogenomics 15(9):1253–1271. doi:10.2217/pgs.14.93
Acknowledgments
This work was supported by the Russian Foundation for Basic Research (project nos. 15-04-05606, 13-0040375-К and 16-34-00200).
Author information
Authors and Affiliations
Corresponding author
Additional information
Anelya Kh. Alieva, Elena V. Filatova, and Anna A. Kolacheva have equal contributions.
Rights and permissions
About this article
Cite this article
Alieva, A.K., Filatova, E.V., Kolacheva, A.A. et al. Transcriptome Profile Changes in Mice with MPTP-Induced Early Stages of Parkinson’s Disease. Mol Neurobiol 54, 6775–6784 (2017). https://doi.org/10.1007/s12035-016-0190-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0190-y