Abstract
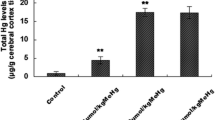
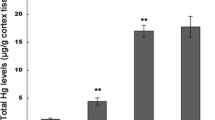
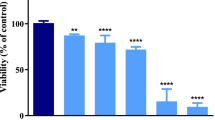
Methylmercury (MeHg) is an extremely dangerous environmental pollutant that induces severe toxic effects in the central nervous system. Neuronal damage plays critical roles mediating MeHg-induced loss of brain function and neurotoxicity. The molecular mechanisms of MeHg neurotoxicity are incompletely understood. The objective of the study is to explore mechanisms that contribute to MeHg-induced neurocyte injuries focusing on neuronal Ca2+ dyshomeostasis and alteration of N-methyl-D-aspartate receptors (NMDARs) expression, as well as oxidative stress in primary cultured cortical neurons. In addition, the neuroprotective effects of memantine against MeHg cytotoxicity were also investigated. The cortical neurons were exposed to 0, 0.01, 0.1, 1, or 2 μM methylmercury chloride (MeHgCl) for 0.5–12 h, or pre-treated with 2.5, 5, 10, or 20 μM memantine for 0.5–6 h, respectively; cell viability and LDH release were then quantified. For further experiments, 2.5, 5, and 10 μM of memantine pre-treatment for 3 h followed by 1 μM MeHgCl for 6 h were performed for evaluation of neuronal injuries, specifically addressing apoptosis; intracellular free Ca2+ concentrations; ATPase activities; calpain activities; expressions of NMDAR subunits (NR1, NR2A, NR2B); NPSH levels; and ROS formation. Exposure of MeHgCl resulted in toxicity of cortical neurons, which were shown as a loss of cell viability, high levels of LDH release, morphological changes, and cell apoptosis. Moreover, intracellular Ca2+ dyshomeostasis, ATPase activities inhibition, calpain activities, and NMDARs expression alteration were observed with 1 μM MeHgCl administration. Last but not least, NPSH depletion and reactive oxygen species (ROS) overproduction showed an obvious oxidative stress in neurons. However, memantine pre-treatment dose-dependently antagonized MeHg-induced neuronal toxic effects, apoptosis, Ca2+ dyshomeostasis, NMDARs expression alteration, and oxidative stress. In conclusion, the cytoprotective effects of memantine against MeHg appeared to be mediated not only via its NMDAR binding properties and Ca2+ homeostasis maintenance but also by indirect antioxidation effects.






Similar content being viewed by others
References
Hong YS, Kim YM, Lee KE (2012) Methylmercury exposure and health effects. J Prev Med Public Health 45:353–363. doi:10.3961/jpmph.2012.45.6.353
Grandjean P, Satoh H, Murata K, et al. (2010) Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect 118:1137–1145. doi:10.1289/ehp.0901757
Liu W, Xu Z, Yang T, et al. (2015) Tea polyphenols protect against methylmercury-induced cell injury in rat primary cultured astrocytes, involvement of oxidative stress and glutamate uptake/metabolism disorders. Mol Neurobiol. doi:10.1007/s12035-015-9161-y
Yin Z, Lee E, Ni M, et al. (2011) Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology 32:291–299. doi:10.1016/j.neuro.2011.01.004
Farina M, Aschner M, Rocha JB (2011) Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol 256:405–417. doi:10.1016/j.taap.2011.05.001
Branco V, Canario J, Holmgren A, et al. (2011) Inhibition of the thioredoxin system in the brain and liver of zebra–seabreams exposed to waterborne methylmercury. Toxicol Appl Pharmacol 251:95–103. doi:10.1016/j.taap.2010.12.005
Liu W, Xu Z, Yang T, et al. (2014) The protective role of tea polyphenols against methylmercury-induced neurotoxic effects in rat cerebral cortex via inhibition of oxidative stress. Free Radic Res 48:849–863. doi:10.3109/10715762.2014.916039
Ceccatelli S, Dare E, Moors M (2010) Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact 188:301–308. doi:10.1016/j.cbi.2010.04.007
Mori N, Yasutake A, Marumoto M, et al. (2011) Methylmercury inhibits electron transport chain activity and induces cytochrome c release in cerebellum mitochondria. J Toxicol Sci 36:253–259. doi:10.2131/jts.36.253
Kumagai Y, Kanda H, Shinkai Y, et al. (2013) The role of the Keap1/Nrf2 pathway in the cellular response to methylmercury. Oxidative Med Cell Longev 2013:848279. doi:10.1155/2013/848279
Feng S, Xu Z, Wang F, et al. (2016) Sulforaphane prevents methylmercury-induced oxidative damage and excitotoxicity through activation of the Nrf2-ARE pathway. Mol Neurobiol. doi:10.1007/s12035-015-9643-y
Yin Z, Milatovic D, Aschner JL, et al. (2007) Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res 1131:1–10. doi:10.1016/j.brainres.2006.10.070
Ahamed M, Akhtar MJ, Siddiqui MA, et al. (2011) Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 283:101–108. doi:10.1016/j.tox.2011.02.010
Nordmann C, Strokin M, Schonfeld P, et al. (2014) Putative roles of Ca(2+) -independent phospholipase A2 in respiratory chain-associated ROS production in brain mitochondria: influence of docosahexaenoic acid and bromoenol lactone. J Neurochem 131:163–176. doi:10.1111/jnc.12789
Quincozes-Santos A, Bobermin LD, Tramontina AC, et al. (2014) Oxidative stress mediated by NMDA, AMPA/KA channels in acute hippocampal slices: neuroprotective effect of resveratrol. Toxicol in Vitro 28:544–551. doi:10.1016/j.tiv.2013.12.021
Xu B, Xu ZF, Deng Y (2009) Effect of manganese exposure on intracellular Ca2+ homeostasis and expression of NMDA receptor subunits in primary cultured neurons. Neurotoxicology 30:941–949. doi:10.1016/j.neuro.2009.07.011
Huo TG, Li WK, Zhang YH, et al. (2015) Excitotoxicity induced by Realgar in the rat hippocampus: the involvement of learning memory injury, dysfunction of glutamate metabolism and NMDA receptors. Mol Neurobiol 51:980–994. doi:10.1007/s12035-014-8753-2
Xu B, Xu Z, Deng Y, et al. (2013) MK-801 protects against intracellular Ca(2+) overloading and improves N-methyl-D-aspartate receptor expression in cerebral cortex of methylmercury-poisoned rats. J Mol Neurosci 49:162–171. doi:10.1007/s12031-012-9926-y
Kaur P, Evje L, Aschner M, et al. (2010) The in vitro effects of Trolox on methylmercury-induced neurotoxicity. Toxicology 276:73–78. doi:10.1016/j.tox.2010.07.006
Abushik PA, Niittykoski M, Giniatullina R, et al. (2014) The role of NMDA and mGluR5 receptors in calcium mobilization and neurotoxicity of homocysteine in trigeminal and cortical neurons and glial cells. J Neurochem 129:264–274. doi:10.1111/jnc.12615
Kania E, Pajak B, Orzechowski A (2015) Calcium homeostasis and ER stress in control of autophagy in cancer cells. Biomed Res Int 2015:352794. doi:10.1155/2015/352794
Aschner M, Syversen T, Souza DO, et al. (2007) Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res 40:285–291. doi:10.1590/S0100-879X2007000300001
Deng Y, Xu Z, Xu B, et al. (2014) Exploring cross-talk between oxidative damage and excitotoxicity and the effects of riluzole in the rat cortex after exposure to methylmercury. Neurotox Res 26:40–51. doi:10.1007/s12640-013-9448-6
Pivovarova NB, Andrews SB (2010) Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J 277:3622–3636. doi:10.1111/j.1742-4658.2010.07754.x
Lu TH, Hsieh SY, Yen CC, et al. (2011) Involvement of oxidative stress-mediated ERK1/2 and p38 activation regulated mitochondria-dependent apoptotic signals in methylmercury-induced neuronal cell injury. Toxicol Lett 204:71–80. doi:10.1016/j.toxlet.2011.04.013
Franco JL, Posser T, Dunkley PR, et al. (2009) Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med 47:449–457. doi:10.1016/j.freeradbiomed.2009.05.013
Xu B, Xu ZF, Deng Y, et al. (2012) Protective effects of MK-801 on methylmercury-induced neuronal injury in rat cerebral cortex: involvement of oxidative stress and glutamate metabolism dysfunction. Toxicology 300:112–120. doi:10.1016/j.tox.2012.06.006
Saxton J, Hofbauer RK, Woodward M, et al. (2012) Memantine and functional communication in Alzheimer’s disease: results of a 12-week, international, randomized clinical trial. J Alzheimers Dis 28:109–118. doi:10.3233/JAD-2011-110947
Parsons CG, Danysz W, Dekundy A, et al. (2013) Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res 24:358–369. doi:10.1007/s12640-013-9398-z
Wang F, Liang W, Lei C, et al. (2015) Combination of HBO and memantine in focal cerebral ischemia: is there a synergistic effect? Mol Neurobiol 52:1458–1466. doi:10.1007/s12035-014-8949-5
Xia P, Chen HS, Zhang D, et al. (2010) Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci 30:11246–11250. doi:10.1523/JNEUROSCI.2488-10.2010
Roth S, Zhang S, Chiu J, et al. (2010) Development of a serum-free supplement for primary neuron culture reveals the interplay of selenium and vitamin E in neuronal survival. J Trace Elem Med Biol 24:130–137. doi:10.1016/j.jtemb.2010.01.007
Koh JY, Choi DW (1987) Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods 20:83–90. doi:10.1016/0165-0270(87)90041-0
Chan E, Yung WH, Baumann KI (1996) Cytoplasmic Ca2+ concentrations in intact Merkel cells of an isolated, functioning rat sinus hair preparation. Exp Brain Res 108:357–366. doi:10.1007/BF00227259
Carfagna MA, Ponsler GD, Muhoberac BB (1996) Inhibition of ATPase activity in rat synaptic plasma membranes by simultaneous exposure to metals. Chem Biol Interact 100:53–65. doi:10.1016/0009-2797(95)03685-7
Nguyen HT, Sawmiller DR, Markov O, et al. (2013) Elevated [Ca2+]i levels occur with decreased calpain activity in aged fibroblasts and their reversal by energy-rich compounds: new paradigm for Alzheimer’s disease prevention. J Alzheimers Dis 37:835–848. doi:10.3233/JAD-131001
Guerguerian AM, Brambrink AM, Traystman RJ, et al. (2002) Altered expression and phosphorylation of N-methyl-D-aspartate receptors in piglet striatum after hypoxiaischemia. Brain Res 104:66–80. doi:10.1016/S0169-328X(02)00285-1
Cheng WW, Lin ZQ, Wei BF, et al. (2011) Singlewalled carbon nanotube induction of rat aortic endothelial cell apoptosis: reactive oxygen species are involved in the mitochondrial pathway. Int J Biochem Cell Biol 43:564–572. doi:10.1016/j.biocel.2010.12.013
Sokolowski K, Falluel-Morel A, Zhou X, et al. (2011) Methylmercury (MeHg) elicits mitochondrial-dependent apoptosis in developing hippocampus and acts at low exposures. Neurotoxicology 32:535–544. doi:10.1016/j.neuro.2011.06.003
Ishida K, Kotake Y, Miyara M, et al. (2013) Involvement of decreased glutamate receptor subunit GluR2 expression in lead-induced neuronal cell death. J Toxicol Sci 38:513–521. doi:10.2131/jts.38.513
Roos D, Seeger R, Puntel R, et al. (2012) Role of calcium and mitochondria in MeHg-mediated cytotoxicity. J Biomed Biotechnol 2012:248764. doi:10.1155/2012/248764
Yan H, Zhang D, Hao S, et al. (2015) Role of mitochondrial calcium uniporter in early brain injury after experimental subarachnoid hemorrhage. Mol Neurobiol 52:1637–1647. doi:10.1007/s12035-014-8942-z
Limke TL, Bearss JJ, Atchison WD (2004) Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor linked pathway. Toxicol Sci 80:60–68. doi:10.1093/toxsci/kfh131
Liu W, Xu Z, Deng Y, et al. (2013) Protective effects of memantine against methylmercury-induced glutamate dyshomeostasis and oxidative stress in rat cerebral cortex. Neurotox Res 24:320–337. doi:10.1007/s12640-013-9386-3
Chuu JJ, Huang ZN, Yu HH, et al. (2008) Attenuation by methyl mercury and mercuric sulfide of pentobarbital induced hypnotic tolerance in mice through inhibition of ATPase activities and nitric oxide production in cerebral cortex. Arch Toxicol 82:343–353. doi:10.1007/s00204-007-0255-9
Huang CF, Hsu CJ, Liu SH, et al. (2008) Neurotoxicological mechanism of methylmercury induced by low-dose and long-term exposure in mice: oxidative stress and down-regulated Na+/K(+)-ATPase involved. Toxicol Lett 176:188–197. doi:10.1016/j.toxlet.2007.11.004
Huang CF, Liu SH, Hsu CJ, et al. (2011) Neurotoxicological effects of low-dose methylmercury and mercuric chloride in developing offspring mice. Toxicol Lett 201:196–204. doi:10.1016/j.toxlet.2010.12.016
Kumar N, Kant R, Maurya PK, et al. (2012) Concentration dependent effect of (−)-epicatechin on Na(+)/K(+)–ATPase and Ca(2+)–ATPase inhibition induced by free radicals in hypertensive patients: comparison with L-ascorbic acid. Phytother Res 26:1644–1647. doi:10.1002/ptr.4624
Liu T, He W, Yan C, et al. (2011) Roles of reactive oxygen species and mitochondria in cadmium-induced injury of liver cells. Toxicol Ind Health 27:249–256. doi:10.1177/0748233710386408
Clausen A, McClanahan T, Ji SG, et al. (2013) Mechanisms of rapid reactive oxygen species generation in response to cytosolic Ca2+ or Zn2+ loads in cortical neurons. PLoS One 8:e83347. doi:10.1371/journal.pone.0083347
Stifanese R, Averna M, De Tullio R, et al. (2010) Adaptive modifications in the calpain/calpastatin system in brain cells after persistent alteration in Ca2+ homeostasis. J Biol Chem 285:631–643. doi:10.1074/jbc.M109.031674
Chimura T, Launey T, Yoshida N (2015) Calpain-mediated degradation of Drebrin by excitotoxicity in vitro and in vivo. PLoS One 10:e0125119. doi:10.1371/journal.pone.0125119
Wang Y, Briz V, Chishti A, et al. (2013) Distinct roles for μ-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J Neurosci 33:18880–18892. doi:10.1523/JNEUROSCI.3293-13.2013
Liu Y, Wong TP, Aarts M, et al. (2007) NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci 27:2846–2857. doi:10.1523/JNEUROSCI.0116-07.2007
Kambe Y, Nakamichi N, Takarada T, et al. (2010) Induced tolerance to glutamate neurotoxicity through downregulation of NR2 subunits of N-methyl-D-aspartate receptors in cultured rat striatal neurons. J Neurosci Res 88:2177–2187. doi:10.1002/jnr.22388
Kaufman AM, Milnerwood AJ, Sepers MD, et al. (2012) Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci 32:3992–4003. doi:10.1523/JNEUROSCI.4129-11.2012
Brewer LD, Thibault O, Staton J, et al. (2007) Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res 1151:20–31. doi:10.1016/j.brainres.2007.03.020
Wang YY, Liu S, Zhang N, et al. (2016) Impaired hippocampal synaptic plasticity and NR2A/2B expression ratio in remifentanil withdrawal rats. Neurotoxicology 53:115–123. doi:10.1016/j.neuro.2016.01.005
Rush T, Liu X, Nowakowski AB, et al. (2012) Glutathione-mediated neuroprotection against methylmercury neurotoxicity in cortical culture is dependent on MRP1. Neurotoxicology 33:476–481. doi:10.1016/j.neuro.2012.03.004
Glaser V, Leipnitz G, Straliotto MR, et al. (2010) Oxidative stress-mediated inhibition of brain creatine kinase activity by methylmercury. Neurotoxicology 31:454–460. doi:10.1016/j.neuro.2010.05.012
Sassi N, Mattarei A, Azzolini M, et al. (2014) Cytotoxicity of mitochondria-targeted resveratrol derivatives: interactions with respiratory chain complexes and ATP synthase. Biochim Biophys Acta 1837:1781–1789. doi:10.1016/j.bbabio.2014.06.010
Gorlach A, Bertram K, Hudecova S, et al. (2015) Calcium and ROS: a mutual interplay. Redox Biol 6:260–271. doi:10.1016/j.redox.2015.08.010
Volbracht C, van Beek J, Zhu C, et al. (2006) Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci 23:2611–2622. doi:10.1111/j.1460-9568.2006.04787.x
Chen CM, Lin JK, Liu SH, et al. (2008) Novel regimen through combination of memantine and tea polyphenol for neuroprotection against brain excitotoxicity. J Neurosci Res 86:2696–2704. doi:10.1002/jnr.21706
Sun D, Chen J, Bao X, et al. (2015) Protection of radial glial-like cells in the hippocampus of APP/PS1 mice: a novel mechanism of memantine in the treatment of Alzheimer’s disease. Mol Neurobiol 52:464–477. doi:10.1007/s12035-014-8875-6
Johnson JW, Glasgow NG, Povysheva NV (2015) Recent insights into the mode of action of memantine and ketamine. Curr Opin Pharmacol 20:54–63. doi:10.1016/j.coph.2014.11.006
Zhang G, Dong Y, Zhang B, et al. (2008) Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci 28:4551–4560. doi:10.1523/JNEUROSCI.5694-07.2008
Acknowledgments
This study was supported by National Natural Science Foundation of China (no. 81172631).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest regarding this paper.
Rights and permissions
About this article
Cite this article
Liu, W., Xu, Z., Yang, T. et al. Memantine, a Low-Affinity NMDA Receptor Antagonist, Protects against Methylmercury-Induced Cytotoxicity of Rat Primary Cultured Cortical Neurons, Involvement of Ca2+ Dyshomeostasis Antagonism, and Indirect Antioxidation Effects. Mol Neurobiol 54, 5034–5050 (2017). https://doi.org/10.1007/s12035-016-0020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0020-2