Abstract
To comprehensively evaluate the therapeutic effects on both functional and structural outcomes, we performed a meta-analysis of preclinical data on stem cell therapy in intracranial hemorrhage, thus providing optimal evidence and instruction for clinical translation. We searched online databases to identify eligible studies based on unmodified stem cell transplantation in intracranial hemorrhage (ICH). From each study, we extracted data regarding neurobehavioral and histological outcomes in order to analyze the comprehensive effective sizes according to the most important clinical parameters (seven indices) and to explore any potential correlation through meta-regression. We analyzed 40 eligible studies including 1021 animals and found a significant improvement in both behavioral and structural outcomes with the median effect size of 1.77 for modified Neurological Severity Score, 1.16 for the modified placement test, 1.82 for the rotarod test, and 1.24 for tissue loss reduction. The meta-regression results revealed that intracerebral administration was the most effective for behavioral and structural recovery post-ICH; mesenchymal stem cells shared comparable therapeutic effects with neural stem cells. Delayed therapy, applied more than 1 week after ICH, showed the greatest improvement of structural outcomes. Stem cell therapy showed significant improvement on behavioral and structural outcomes of ICH animals with relatively large effect sizes. However, the practical efficacy of the therapy is likely to be lower considering poor study quality and non-negligible publication bias. Further, future research should interpret animal results cautiously considering the limited internal and external validity when referring to the design of both animal studies and clinical trials.
Similar content being viewed by others
Introduction
Intracerebral hemorrhage (ICH), which results from rupture of blood vessels in the brain, remains a public health concern because it leads to high rates of mortality and disability in adults [1]. Hematoma expansion and brain edema are two important complications that might occur during the acute and subacute phases of ICH; both are known to exacerbate brain injury [2, 3]. Management of ICH is largely carried out via mechanical removal of the hematoma, pharmacological prevention of edema formation, and reduction in intracranial pressure. The intent of all of these methods is to limit further brain injury and associated complications. Unfortunately, compared with ischemic stroke, ICH has received less research attention, and, to date, no currently available medical therapy has shown a consistent or unambiguous benefit in functional outcomes after ICH [4, 5].
Stem cells—including embryonic stem cells (ESCs), somatic stem cells, and induced pluripotent stem cells (iPSCs)—are characterized by their capacity for self-renewal and multiple differentiation. Mesenchymal stem cells (MSCs) have long been used in preclinical and clinical research [6, 7], and stem cell therapy has become one of the most promising strategies for the treatment of comprehensive human diseases, such as ischemic heart disease, autoimmune diseases, and neurological disorders [8–11]. The general intent of stem cell therapy is to replace lost cells or restore the function of damaged tissues by introducing with the capacity of secreting multiple growth factors, cytokines, and neurotrophins. However, the underlying mechanisms are far more complex and still not fully understood at present [12–14].
A number of reports on stem cell transplantation in ICH animal models indicate improved neurobehaviors or attenuation of the hematoma [15–17]. Among various stem cells, MSCs and neural stem cells (NSCs) are the most widely used and offer great promise in ICH repair. However, the administration dose, route, time interval, cell source, type, manipulation, and quality score in each study are so divergent that the overall therapeutic effect is difficult to evaluate. Consequently, the optimal pattern of cell therapy remains unclear [18]. To clarify the current situation and future research directions in cell therapy as a treatment for ICH, we collected data from all relevant studies and performed a meta-analysis to quantify both the functional and structural efficacy of stem cell therapy. Among these studies, the most widely used empirical tests to assess behavioral outcome of ICH include the modified neurological severity score (mNSS), modified limb placement test (mLPT), and rotarod test [19, 20]. The studies included in the meta-analysis are graded strictly according to Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Stroke (CAMARADES) checklists [21].
Methods
Search Strategy
Studies of stem cell-based therapy for ICH animal models were retrieved from PubMed and Medline through April 2015 using the following search terms: (progenitor OR stem OR bone marrow OR mesenchymal OR haematopoietic) AND (basal ganglion hemorrhage OR brain hemorrhage OR brain ventricle hemorrhage OR cerebellum hemorrhage OR intracranial hemorrhage OR hemorrhage stroke). Two researchers worked independently on these searches (Yang Hu and Na Liu). Retrieved articles and abstracts, including secondary references, were thoroughly scanned and reviewed by the two researchers, either. Eligible studies were reviewed in duplicate to determine whether or not to be included in the meta-analysis.
Inclusion and Exclusion Criteria
The meta-analysis included controlled studies that compared neurobehavioral outcomes—with or without structural outcomes—in wild-type (nontransgenic) animal groups. In these studies, ICH was induced using autologous blood or collagenase injection, after which subjects were treated with allogeneic or autologous stem cells and a placebo (saline, culture medium, or similar vehicle). The meta-analysis excluded studies that used nontraumatic models of hemmorrhagic injury or individual comparisons that were not reported or from which we could not calculate the number of animals, the mean outcome, or the variance in each group. We also excluded studies that used substantially manipulated stem cells, including cells differentiated into mature neural cells, co-transplanted with other stem cells, or transfected with genes other than labeling or tracing markers.
Data Collection
The following items from the eligible studies were independently extracted by the two researchers: general study information (first author, publication date), ICH model, cell characteristics (cell type, source, dose, delivery route), recipient animal species, functional outcome (neurobehavioral score measured on any scale, modified limb placement test, rotarod test), structural outcome (brain water content or tissue loss reduction), and study quality index.
From each experiment, the researchers extracted, without exception, all available data from reported outcomes available, text, and graphs. When only graphic presentations were available, values for mean and standard deviation (SD) were obtained via calibrating images using GetData Graph Digitizer software. If the study included more than one experimental group differentiated by delivery time or cell number that was compared against a common control group, these parallel groups would be included separately as independent experiments and the control group size divided equally among the numbers of treatment groups. If the SD was not directly reported, it was calculated by multiplying the reported standard error (SE) by the square root of the group size. Where functional outcome was measured at different times, only the most recent one was extracted.
Methodological Quality of Studies
The quality of each experiment was assessed according to the CAMARADES checklists, which consist of the following: (1) publication in a peer-reviewed journal, (2) control of animals’ temperature, (3) randomized treatment allocation, (4) treatment allocation concealment, (5) blind assessment of outcome, (6) avoidance of anesthetics with known marked intrinsic neuroprotective activity such as ketamine, (7) reporting of a sample size calculation, (8) statement of compliance with regulatory requirements, (9) statement of potential conflicts of interest, and (10) use of animals with relevant comorbidities.
Statistical Analyses
Effect size was calculated to be the absolute difference with 95 % confidence interval (CI) between stem cell treated animals and comparable controls. For the rotarod test, in contrast to the other three measures, outcome values were multiplied by −1 because the value was positively correlated with its outcome. The DerSimonian-Laird random meta-analysis model and Hedges calculation were adopted to determine a comprehensive estimation of effect size with standard mean differences, and meta-analysis was performed using Stata software (version 12.1). Generally, an effect size of 0.2 was defined as a small effect, 0.5 as medium and 0.8 as large. A probability value of P < 0.05 was considered statistically significant. Seven intriguing clinical parameters were used to stratify the effect size: cell source (autologous, allogeneic, or xenogeneic); cell type (NSCs, MSCs, or other stem cells); cell dose (<1E6, 1E6–5E6, >5E6); delivery time (0–8 h, 24 h, 1–7 days, or >7 days); randomization; blind review by operator; and total quality score. Meta-regression and pre-specified subgroup analysis were used to explore the potential relationships between mNSS and tissue loss and the aforementioned parameters. Publication bias was examined using funnel plots, and significant publication bias was assessed using Egger regression. If necessary, any non-negligible bias would be corrected using the Duval and Tweedie trim-and-fill approach.
Results
Study Characteristics
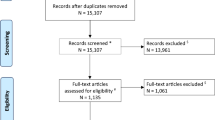
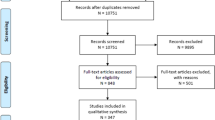
Electronic searching identified 440 articles in PubMed and 173 articles in EMBASE. From among these, 40 eligible studies containing 589 cell treatment animals and 432 comparable controls were eventually included in our meta-analysis (Fig. 1). Twenty of these studies demonstrated improvement in mNSS, and seven of the 11 studies reported decreased tissue loss. The vast majority of the studies (62.5 %) used MSCs derived from bone marrow, adipose tissue, or umbilical blood, and, except for two studies that used iPSCs, the remaining studies used NSCs. In 33 of the 40 studies, a collagenase-induced ICH model was adopted, while the remaining studies adopted an autologous blood-induced ICH model.
Quality Score
The quality scores varied from 1 to 7, with a mean value of 4.45. According to our statistical results, all the articles were published in a peer-reviewed journal; 37.5 % described control of temperature, 75 % declared randomization to treatment group, 60 % stressed blind assessment of outcome, 50 % avoided usage of ketamine as anesthetics, 90 % claimed compliance with animal welfare regulations, and 30 % stated a conflict of interest (Table 1; SI File).
Meta-analysis
Overall, use of stem cell therapy showed an improvement in both functional and structural outcomes post-ICH, and all four effect sizes were statistically significant (1.77 for mNSS, 1.16 for MLPT, 1.82 for RR, and 1.24 for tissue loss reduction) (Fig. 2). Observed heterogeneity was higher than what would be expected from sampling error alone and could not be ignored (τ 2 = 0.6777, I 2 = 61 % for mNSS; τ 2 = 0.1751, I 2 = 42.1 % for MLPT; τ 2 = 1.6451, I 2 = 86.7 % for RR; τ 2 = 0.1833, I 2 = 35.4 % for tissue loss reduction). For the two outcomes with the largest amount of published data—mNSS and tissue loss reduction—meta-regression was used to explore potential contributions to heterogeneity of the parameters mentioned above.
Meta-regression for Functional and Structural Outcomes
For mNSS, publication year remained the only significant predictor (P = 0.036); the more recently the research was conducted, the larger the effect sizes were (Fig. 3a). For tissue loss reduction, administration route and time remained as significant predictors (P = 0.005 for administration route; P = 0.002 for administration time); in fact, intracerebral (ICV) injection was demonstrated to be the most effective route for improvement of both mNSS and tissue loss reduction, more so than intravenous (IV) and intra-arterial (IA) injections. In tissue loss reduction, cell therapy initiated more than 1 week post-ICH showed the greatest difference, followed next by cell therapy initiated within 1 week post-ICH, and then therapy initiated with 24 h (mean effect size 3.027 vs 1.270 vs 0.942; P = 0.002; Fig. 3b). None of the other clinical parameters were predictors for effect size, nor were any of the three quality parameters (total quality score, randomization, and allocation concealment). Additionally, subgroup analysis revealed that MSCs shared a comparable effect size with NSCs, as did allogenic cells with xenogenic cells (Tables 2 and 3).
Publication Bias
Visual inspection of funnel plots of mNSS and MLPT revealed asymmetry. This was consistent with the results from the Egger test, which suggested prominent publication bias to the left of the estimate (P = 0.004 for mNSS; P = 0.001 for MLPT). However, after correcting for the bias, the pooled analysis incorporating the hypothetical studies remained statistically significant, and all four calibrated effect sizes exceed 1 (1.367 for mNSS and 1.053 for MLPT).
Discussion
This meta-analysis including 40 studies and 1021 animals suggests the following: (1) ICH animals benefited greatly from stem cell therapy, with treatment using both NSCs and MSCs exhibiting statistically significant improvement in functional and structural outcomes. (2) ICV proved to be the most effective administration route compared with IV or IA to improve mNSS and decrease tissue loss. (3) Allogenic and xenogenic cells exhibited similarly beneficial effect size for mNSS, although the former facilitated lesion site recovery to a larger extent. (4) Intervention time was positively correlated with effect size in tissue loss reduction but not in mNSS. (5) Stem cell-based therapy shows promise in treating ICH animals based on our study, but determining the efficacy is inevitably confounded by poor study quality and publication bias. Therefore, our conclusions should be tested in more rigorously designed studies and carefully interpreted in relation to the design of future clinical translation or animal studies.
The categories of stem cells already used for ICH animals are NSCs [16, 17], ESCs [22], human MSCs [23], human bone marrow stromal cells (HBMSCs) [24], adipose-derived stem cells [25], human umbilical cord blood cells (HUCBCs), and human iPSCs [26]. Our meta-analysis included all but ESCs, with the majority being MSCs derived from various tissues. Our pre-specified subgroup analysis stratified by cell type showed that MSCs had a greater positive impact on mNSS, while NSCs were more effective in decreasing tissue loss. Although post hoc meta-regression detected no correlation between cell type and effect size, this distinction between the two cells types might be explained by their different therapeutic mechanisms. To be specific, because of the unique capacity of NSCs to differentiate into functional neural cells, reduced tissue loss might result from donor NSCs replacing damaged neurons, although very few studies have rigorously examined the electrophysiological and transmitter synthesis function of transplanted NSCs in vivo [27, 28]. But again, this is likely the result of NSC secretion of several cytokines after ICH, which, as recent studies have shown, attenuate systemic inflammatory response [10, 29, 30]. Even with these recent studies, this unique mechanism requires further exploration in future research work. Compared with NSCs, the effectiveness of MSCs as documented in models of neurological disease is attributed mainly to the cells’ ability to secrete various neurotrophins, cytokines, or growth factors, including brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF), all of which possibly contribute to the functional recovery of the subventricular zone (SVZ) in the adult central nervous system [23, 31, 32]. The number of transplanted MSCs that express physical and biochemical characteristics of neural cells is extremely low, so virtually none of them are able to be integrated into functional neural circuitry [33]. Nevertheless, the underlying mechanisms are far more complex and still not fully understood at present. Given the current state of research, we find that MSCs share comparable therapeutic effects with NSCs in ICH, which challenges the obsolete view that NSCs might be considered the most appropriate cells for treating nervous system diseases like ICH. Therefore, MSCs seem to be a promising cell source for future clinical application because of their similarity to NSCs in their effects, and yet they require a less invasive and delicate isolation procedures than NSCs [34].
Of additional concern are the immunological issues commonly associated with the process of allogenic or xenogenic cell transplantation. Once exogenous cells trigger an intrinsic immune rejection in vivo, the transplanted cells will be eliminated by the activated immune system, a process that not only destroys the cell functions, but also causes damage to the hosts [35, 36]. Considering that majority of cells included in our analysis were MSCs, which can exert overt regulation on the host’s immune system, no obvious immune rejections were reported, even in the xenogenic cell transplantation group not simultaneously treated with immunosuppressants. In our subgroup analysis, the difference in effect size between allogenic and xenogenic cells was not statistically significant (P > 0.05).
Administration route and dose are typically the focus if cell therapy is applied in clinical situations. In our analysis, the intracerebral route seems to have a distinct advantage over both the IV and intracarotid routes. Compared with ICV, either IV or IA is relatively less invasive and more convenient to manipulate. Temporarily disregarding the inconvenience of ICV, it does show superiorities over IV route in that it can rapidly and directly target the lesion site while avoiding spleen phagocytosis and retardation of brain blood barrier retention [37–39]. More importantly, it is possible to combine cell therapy administered through ICV with hematoma evacuation after ICH [40, 41]. At the same time, cell viability might decrease significantly once the cells are exposed directly via ICV to a heavily inflammatory local environment. Therefore, exploring innovative and effective alternative delivery routes such as intranasal delivery might be a useful future research direction. The majority of the included studies tended to initiate the cell therapy at 24 h post-ICH, followed by therapy initiation within 1 week post-ICH; only four exceeded more than 1 month before beginning treatment, and two of those did not begin treatment until 2 months post-ICH. We found a positive correlation between intervention time and structural effect size, an observation that might be explained by the fact that a substantial number of studies conducted a final structural assessment before reaching the plateau recovery phase. However, it is important to keep in mind that subgroup analyses can only generate hypotheses rather than confirming them.
Assessment of the methodological quality of animal studies should follow strict criteria regarding clinical trials because of the potential that the design of these trials might influence their results [21]. The average quality score according to the CAMARADES list was lower than we had expected. The percentage of descriptive randomization and blindness were 75 and 60, respectively, and none of the studies explicitly stated the specific procedures followed in conducting the experiments. Additionally, half of the studies adopted ketamine as the anesthetic regardless of its well-known neuroprotective effects. Intriguingly, randomization, blinding, and total quality score were not significant predictors for effect size, which can be partially attributed to the ambiguous description of the methods. As a result, we could not predict the effect size for subsequent studies based on quality scores. To reduce the risk of bias and given this evidence of the poor reporting of measures, we encourage future research to report both detailed methodology and measures performance in the field. Both Funnel plots and Egger tests detected obvious publication bias, so a trim-and-fill approach was adopted to correct the bias, although the modified effect sizes were still remarkable. As might be expected, studies reporting more positive outcomes are more likely to be accepted for publication, especially in animal studies.
There is significant work to be done when it comes to clinical translation [42]. First of all, the study subjects are primarily rats, which share limited similarities with human beings in anatomical and biochemical properties, unlike porcines or primates, which can mimic human pathological process more vividly and precisely [43, 44]. Thus, because the neurobehavioral outcomes for rats cannot be directly extended to human beings, more reliable results could be obtained by using multiple species. Secondly, although ICH is generally the result of ruptured vessels affected by hypertension-related degenerative changes or the natural course of cerebral amyloid angiopathy, current study models are almost entirely based on healthy animals without any chronic comorbidities, letting alone hypertension [45]. Furthermore, the majority of ICH patients are elderly, while study animals are selected from a fixed age group with a high proportion of youngsters. In terms of the meta-analysis itself, the internal and external validity are obviously confounded by poor study quality and non-negligible publication bias. Consequently, the efficacy of stem cell therapy for ICH will be amplified to some extent because studies that remain more often contain neutral or negative data.
Although several reviews and meta-analyses focusing on cell therapy for neurological diseases have been published, this is the first meta-analysis to concentrate specifically on stem cells used for ICH in animal models [46, 47]. Although the obvious effect size for ischemic stroke of MSCs alone or for all stem cells has been emphasized, the pathophysiological features of ischemic and hemorrhagic stroke diverge in many aspects, so great caution should be taken when comparing findings for each disease [48].
According to our study, stem cell-based therapies may offer promise in treating ICH, and, the therapeutic effect of stem cells in this application seems obvious based on preclinical research. However, future research should interpret animal results cautiously considering the limited internal and external validity when referring to the design of both animal studies and clinical trials.
References
Qureshi AI, Mendelow AD, Hanley DF (2009) Intracerebral haemorrhage. Lancet 373(9675):1632–1644. doi:10.1016/S0140-6736(09)60371-8
Balami JS, Buchan AM (2012) Complications of intracerebral haemorrhage. Lancet Neurol 11(1):101–118. doi:10.1016/S1474-4422(11)70264-2
Ovesen C, Christensen AF, Krieger DW, Rosenbaum S, Havsteen I, Christensen H (2014) Time course of early postadmission hematoma expansion in spontaneous intracerebral hemorrhage. Stroke J Cereb Circ 45(4):994–999. doi:10.1161/STROKEAHA.113.003608
Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN et al (2015) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American heart association/american stroke association. Stroke J Cereb Circ. doi:10.1161/STR.0000000000000069
Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE, Collaboration V (2011) Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 76(14):1238–1244. doi:10.1212/WNL.0b013e3182143317
Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9(1):11–15. doi:10.1016/j.stem.2011.06.008
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8(9):726–736. doi:10.1038/nri2395
Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, Hagege AA, Menasche P (2003) Transplantation of autologous fresh bone marrow into infarcted myocardium: a word of caution. Circulation 108(Suppl 1):II247–II252. doi:10.1161/01.cir.0000089040.11131.d4
Kaigler D, Pagni G, Park CH, Braun TM, Holman LA, Yi E, Tarle SA, Bartel RL et al (2013) Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant 22(5):767–777. doi:10.3727/096368912X652968
Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH et al (2008) Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain J Neurol 131(Pt 3):616–629. doi:10.1093/brain/awm306
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J et al (2010) Hematopoietic stem cell transplantation: a global perspective. JAMA 303(16):1617–1624. doi:10.1001/jama.2010.491
Hildreth CJ, Burke AE, Glass RM (2009) JAMA patient page. Hematopoietic stem cell transplantation. JAMA 302(3):340. doi:10.1001/jama.302.3.340
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K et al (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15(1):42–49. doi:10.1038/nm.1905
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P et al (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5(1):54–63. doi:10.1016/j.stem.2009.05.003
Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, Oh WI, Park WS (2013) Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke J Cereb Circ 44(2):497–504. doi:10.1161/strokeaha.112.679092
Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK (2003) Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke J Cereb Circ 34(9):2258–2263. doi:10.1161/01.str.0000083698.20199.1f
Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, Ko Y, Jeong SW et al (2007) Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells (Dayton, Ohio) 25(5):1204–1212. doi:10.1634/stemcells.2006-0409
Cordeiro MF, Horn AP (2015) Stem cell therapy in intracerebral hemorrhage rat model. World J Stem Cells 7(3):618–629. doi:10.4252/wjsc.v7.i3.618
Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M (2001) Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci 189(1–2):49–57
Puurunen K, Jolkkonen J, Sirvio J, Haapalinna A, Sivenius J (2001) An alpha(2)-adrenergic antagonist, atipamezole, facilitates behavioral recovery after focal cerebral ischemia in rats. Neuropharmacology 40(4):597–606
Macleod MR, O’Collins T, Howells DW, Donnan GA (2004) Pooling of animal experimental data reveals influence of study design and publication bias. Stroke J Cereb Circ 35(5):1203–1208. doi:10.1161/01.STR.0000125719.25853.20
Nonaka M, Yoshikawa M, Nishimura F, Yokota H, Kimura H, Hirabayashi H, Nakase H, Ishizaka S, Wanaka A, Sakaki T (2004) Intraventricular transplantation of embryonic stem cell-derived neural stem cells in intracerebral hemorrhage rats. Neurological research 26(3):265–272. doi:10.1179/016164104225014049
Seyfried D, Ding J, Han Y, Li Y, Chen J, Chopp M (2006) Effects of intravenous administration of human bone marrow stromal cells after intracerebral hemorrhage in rats. J Neurosurg 104(2):313–318. doi:10.3171/jns.2006.104.2.313
Zhang H, Huang Z, Xu Y, Zhang S (2006) Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol Res 28(1):104–112. doi:10.1179/016164106x91960
Chen J, Tang YX, Liu YM, Chen J, Hu XQ, Liu N, Wang SX, Zhang Y et al (2012) Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther 18(10):847–854. doi:10.1111/j.1755-5949.2012.00382.x
Qin J, Song B, Zhang H, Wang Y, Wang N, Ji Y, Qi J, Chandra A et al (2013) Transplantation of human neuro-epithelial-like stem cells derived from induced pluripotent stem cells improves neurological function in rats with experimental intracerebral hemorrhage. Neurosci Lett 548:95–100. doi:10.1016/j.neulet.2013.05.007
Le Douarin NM, Creuzet S, Couly G, Dupin E (2004) Neural crest cell plasticity and its limits. Development (Cambridge, England) 131(19):4637–4650. doi:10.1242/dev.01350
Bjorklund A, Lindvall O (2000) Cell replacement therapies for central nervous system disorders. Nat Neurosci 3(6):537–544. doi:10.1038/75705
Cusimano M, Biziato D, Brambilla E, Donega M, Alfaro-Cervello C, Snider S, Salani G, Pucci F et al (2012) Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain J Neurol 135(Pt 2):447–460. doi:10.1093/brain/awr339
Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A et al (2005) Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 436(7048):266–271. doi:10.1038/nature03889
Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ et al (2002) Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59(4):514–523
Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F et al (2007) Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol 61(3):219–227. doi:10.1002/ana.21076
Li Y, Chen J, Wang L, Lu M, Chopp M (2001) Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56(12):1666–1672
Kim JM, Lee ST, Chu K, Jung KH, Song EC, Kim SJ, Sinn DI, Kim JH et al (2007) Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res 1183:43–50. doi:10.1016/j.brainres.2007.09.005
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B et al (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371(9624):1579–1586. doi:10.1016/S0140-6736(08)60690-X
Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X et al (2010) Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res 20(5):510–518. doi:10.1038/cr.2010.44
Kopen GC, Prockop DJ, Phinney DG (1999) Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A 96(19):10711–10716
Lee HJ, Lim IJ, Lee MC, Kim SU (2010) Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res 88(15):3282–3294. doi:10.1002/jnr.22474
Wang Z, Cui C, Li Q, Zhou S, Fu J, Wang X, Zhuge Q (2011) Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J Cell Mol Med 15(12):2624–2633. doi:10.1111/j.1582-4934.2011.01259.x
Juvela S, Kase CS (2006) Advances in intracerebral hemorrhage management. Stroke J Cereb Circ 37(2):301–304. doi:10.1161/01.STR.0000200445.68303.25
Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, Dong Q, Guo J et al (2012) Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke J Cereb Circ 43(11):2923–2930. doi:10.1161/STROKEAHA.112.667535
Kirkman MA, Allan SM, Parry-Jones AR (2011) Experimental intracerebral hemorrhage: avoiding pitfalls in translational research. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 31(11):2135–2151. doi:10.1038/jcbfm.2011.124
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 87(1):179–197. doi:10.1016/j.pbb.2007.04.015
Chopp M, Steinberg GK, Kondziolka D, Lu M, Bliss TM, Li Y, Hess DC, Borlongan CV (2009) Who’s in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant 18(7):691–693. doi:10.3727/096368909X470883
Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, Group S (2009) Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke J Cereb Circ 40(6):2244–2250. doi:10.1161/STROKEAHA.108.541128
Frantzias J, Sena ES, Macleod MR, Al-Shahi Salman R (2011) Treatment of intracerebral hemorrhage in animal models: meta-analysis. Ann Neurol 69(2):389–399. doi:10.1002/ana.22243
Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR (2012) Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. Int J Stroke Off J Int Stroke Soc 7(7):582–588. doi:10.1111/j.1747-4949.2012.00797.x
Vu Q, Xie K, Eckert M, Zhao W, Cramer SC (2014) Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology 82(14):1277–1286. doi:10.1212/WNL.0000000000000278
Acknowledgments
This study was supported by funds provided by National Natural Science Foundation of China (81171089, 81471201 and 81170322) and the Wuhan Science and Technology bureau Foundation of China (2013060602010240).
Conflicts of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 149 kb)
Glossary
- CAMARADES
-
Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Stroke
- ICH
-
Intracerebral hemorrhage
- IPS
-
Induced pluripotent stem cell
- MLPT
-
Modified limb placement test
- mNSS
-
Modified neurological severity score
- MSC
-
Mesenchymal stem cell
- NSC
-
Neural stem cell
- RR
-
Rotarod test
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hu, Y., Liu, N., Zhang, P. et al. Preclinical Studies of Stem Cell Transplantation in Intracerebral Hemorrhage: a Systemic Review and Meta-Analysis. Mol Neurobiol 53, 5269–5277 (2016). https://doi.org/10.1007/s12035-015-9441-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9441-6