Abstract
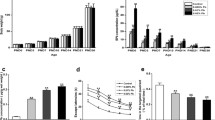
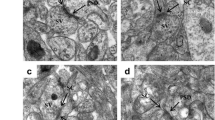
Lead (Pb) is an environmental neurotoxic metal. Pb exposure may cause neurobehavioral changes, such as learning and memory impairment, and adolescence violence among children. Previous animal models have largely focused on the effects of Pb exposure during early development (from gestation to lactation period) on neurobehavior. In this study, we exposed Sprague-Dawley rats during the juvenile stage (from juvenile period to adult period). We investigated the synaptic function and structural changes and the relationship of these changes to neurobehavioral deficits in adult rats. Our results showed that juvenile Pb exposure caused fear-conditioned memory impairment and anxiety-like behavior, but locomotion and pain behavior were indistinguishable from the controls. Electrophysiological studies showed that long-term potentiation induction was affected in Pb-exposed rats, and this was probably due to excitatory synaptic transmission impairment in Pb-exposed rats. We found that NMDA and AMPA receptor-mediated current was inhibited, whereas the GABA synaptic transmission was normal in Pb-exposed rats. NR2A and phosphorylated GluR1 expression decreased. Moreover, morphological studies showed that density of dendritic spines declined by about 20 % in the Pb-treated group. The spine showed an immature form in Pb-exposed rats, as indicated by spine size measurements. However, the length and arborization of dendrites were unchanged. Our results suggested that juvenile Pb exposure in rats is associated with alterations in the glutamate receptor, which caused synaptic functional and morphological changes in hippocampal CA1 pyramidal neurons, thereby leading to behavioral changes.









Similar content being viewed by others
References
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Nevin R (2009) Trends in preschool lead exposure, mental retardation, and scholastic achievement: association or causation. Environ Res 109(3):301–310
Carpenter DO, Nevin R (2010) Environmental causes of violence. Physiol Behav 99(2):260–268
Hunt PS, Jacobson SE, Torok EJ (2009) Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: dose-response and timing effects. Alcohol 43(6):465–474
Jaako-Movits K, Zharkovsky T, Romantchik O et al (2005) Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. Int J Dev Neurosci 23(7):627–635
Barbosa AC, Kim MS, Ertunc M et al (2008) MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci U S A 105(27):9391–9396
Trinchese F, Fa M, Liu S et al (2008) Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest 118(8):2796–2807
Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429(6993):761–6
Yuste R, Bonhoeffer T (2001) Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 24:1071–1089
Cingolani LA, Goda Y (2008) Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci 9(5):344–356
Routh BN, Johnston D, Harris K, Chitwood RA (2009) Anatomical and electrophysiological comparison of CA1 pyramidal neurons of the rat and mouse. J Neurophysiol 102(4):2288–2302
Megias M, Emri Z, Freund TF, Gulyas AI (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102(3):527–540
Lang S, Kroll A, Lipinski SJ et al (2009) Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci 29(4):823–832
Gasparini S, Saviane C, Voronin LL, Cherubini E (2000) Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release. Proc Natl Acad Sci U S A 97(17):9741–9746
Bouchard MF, Bellinger DC, Weuve J et al (2009) Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Arch Gen Psychiatry 66(12):1313–1319
Bannerman DM, Sprengel R, Sanderson DJ et al (2014) Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci 15(3):181–192
Turrigiano GG, Nelson SB (2004) Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5(2):97–107
Braga MF, Pereira EF, Albuquerque EX (1999) Nanomolar concentrations of lead inhibit glutamatergic and GABAergic transmission in hippocampal neurons. Brain Res 826(1):22–34
Tang M, Luo L, Zhu D et al (2009) Muscarinic cholinergic modulation of synaptic transmission and plasticity in rat hippocampus following chronic lead exposure. Naunyn Schmiedebergs Arch Pharmacol 379(1):37–45
Maffei A, Nelson SB, Turrigiano GG (2004) Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci 7(12):1353–1359
Liu MC, Liu XQ, Wang W et al (2012) Involvement of microglia activation in the lead induced long-term potentiation impairment. PLoS One 7(8), e43924
Domercq M, Vazquez-Villoldo N, Matute C (2013) Neurotransmitter signaling in the pathophysiology of microglia. Front Cell Neurosci 7:49
Liu M, Li J, Dai P et al (2015) Microglia activation regulates GluR1 phosphorylation in chronic unpredictable stress-induced cognitive dysfunction. Stress 18(1):96–106
Guilarte TR, McGlothan JL (1998) Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res 790(1-2):98–107
Neal AP, Worley PF, Guilarte TR (2011) Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology 32(2):281–289
Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6(2):136–143
Benke TA, Luthi A, Isaac JT, Collingridge GL (1998) Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393(6687):793–797
Luthi A, Wikstrom MA, Palmer MJ et al (2004) Bi-directional modulation of AMPA receptor unitary conductance by synaptic activity. BMC Neurosci 5:44
Engert F, Bonhoeffer T (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399(6731):66–70
Maletic-Savatic M, Malinow R, Svoboda K (1999) Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283(5409):1923–1927
Tian L, Stefanidakis M, Ning L et al (2007) Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol 178(4):687–700
Ultanir SK, Kim JE, Hall BJ, Deerinck T, Ellisman M, Ghosh A (2007) Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci U S A 104(49):19553–19558
Chen BS, Thomas EV, Sanz-Clemente A, Roche KW (2011) NMDA receptor-dependent regulation of dendritic spine morphology by SAP102 splice variants. J Neurosci 31(1):89–96
Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A (2000) Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci 3(9):887–894
Hu F, Xu L, Liu ZH, Ge MM, Ruan DY, Wang HL (2014) Developmental lead exposure alters synaptogenesis through inhibiting canonical Wnt pathway in vivo and in vitro. PLoS One 9(7), e101894
Munoz FJ, Godoy JA, Cerpa W, Poblete IM, Huidobro-Toro JP, Inestrosa NC (2014) Wnt-5a increases NO and modulates NMDA receptor in rat hippocampal neurons. Biochem Biophys Res Commun 444(2):189–194
Catterall WA, Few AP (2008) Calcium channel regulation and presynaptic plasticity. Neuron 59(6):882–901
Schulz PE, Cook EP, Johnston D (1994) Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci 14(9):5325–5337
Acknowledgments
This work was supported by the Key National Scientific Foundation of China (#81230063); National Basic Research Program of China (973 Program, #2012CB525002); National Key Technology Support Program (#2014BAI12B04); National Scientific Foundation of China (#81302451, #81273101, #81472942, and #81402650); Program for Changjiang Scholars (T2011153) and Innovative Research Team in University (PCSIRT); and Shaanxi science and technology coordinating innovative project (2011KTCL03-19).
Conflict of Interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, T., Guan, RL., Liu, MC. et al. Lead Exposure Impairs Hippocampus Related Learning and Memory by Altering Synaptic Plasticity and Morphology During Juvenile Period. Mol Neurobiol 53, 3740–3752 (2016). https://doi.org/10.1007/s12035-015-9312-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9312-1