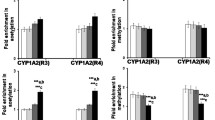
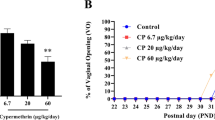
Abstract
Oral administration of low doses of cypermethrin to pregnant Wistar rats led to a dose-dependent differences in the induction of xenobiotic-metabolizing cytochrome P450s (CYPs) messenger RNA (mRNA) and protein in brain regions isolated from the offsprings postnatally at 3 weeks that persisted up to adulthood. Similar alterations were observed in the expression of rate-limiting enzymes of neurotransmitter synthesis in brain regions of rat offsprings. These persistent changes were associated with alterations in circulating levels of growth hormone (GH), cognitive functions, and accumulation of cypermethrin and its metabolites in brain regions of exposed offsprings. Though molecular docking studies failed to identify similarities between the docked conformations of cypermethrin with CYPs and neurotransmitter receptors, in silico analysis identified regulatory sequences of CYPs in the promoter region of rate-limiting enzymes of neurotransmitter synthesis. Further, rechallenge of the prenatally exposed offsprings at adulthood with cypermethrin (p.o. 10 mg/kg × 6 days) led to a greater magnitude of alterations in the expression of CYPs and rate-limiting enzymes of neurotransmitter synthesis in different brain regions. These alterations were associated with a greater magnitude of decrease in the circulating levels of GH and cognitive functions in rechallenged offsprings. Our data has led us to suggest that due to the immaturity of CYPs in fetus or during early development, even the low-level exposure of cypermethrin may be sufficient to interact with the CYPs, which in turn affect the neurotransmission processes and may help in explaining the developmental neurotoxicity of cypermethrin.











Similar content being viewed by others
Change history
26 June 2019
The original version of this article unfortunately contained an error at Fig.��10.
26 June 2019
The original version of this article unfortunately contained an error at Fig.��10.
References
Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML (2002) Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology 171(1):3–59
Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi JS et al (2009) Evidence for a separate mechanism of toxicity for the type I and the type II pyrethroid insecticides. Neurotoxicology 30(Suppl 1):S17–S31
Husain R, Malaviya M, Seth PK, Husain R (1994) Effect of deltamethrin on regional brain polyamines and behaviour in young rats. Pharmacol Toxicol 74(6):211–215
Rao GV, Jagannatha Rao KS (1995) Modulation in acetylcholinesterase of rat brain by pyrethroids in vivo and an in vitro kinetic study. J Neurochem 65(5):2259–2266
Singh A, Mudawal A, Shukla RK, Yadav S, Khanna VK, Sethumadhavan R, Parmar D (2014) Effect of gestational exposure of cypermethrin on postnatal development of brain cytochrome P450 2D1 and 3A1 and neurotransmitter receptors. Mol Neurobiol 1–16
Scollon EJ, Starr JM, Godin SJ, DeVito MJ, Hughes MF (2009) In vitro metabolism of pyrethroid pesticides by rat and human hepatic microsomes and cytochrome p450 isoforms. Drug Metab Dispos 37(1):221–228
Anadon A, Martinez-Larranaga MR, Fernandez-Cruz ML, Diaz MJ, Fernandez MC, Martinez MA (1996) Toxicokinetics of deltamethrin and its 4′-HO-metabolite in the rat. Toxicol Appl Pharmacol 141(1):8–16
Hutson DH, Gaughan LC, Casida JE (1981) Metabolism of the cis- and trans-isomers of cypermethrin in mice. Pestic Sci 12(4):385–398
Johri A, Yadav S, Singh RL, Dhawan A, Ali M, Parmar D (2006) Long lasting effects of prenatal exposure to deltamethrin on cerebral and hepatic cytochrome P450s and behavioral activity in rat offsprings. Eur J Pharmacol 544(1–3):58–68
Singh A, Yadav S, Srivastava V, Kumar R, Singh D, Sethumadhavan R, Parmar D (2013) Imprinting of cerebral and hepatic cytochrome P450s in rat offsprings exposed prenatally to low doses of cypermethrin. Mol Neurobiol 48(1):128–140
Parkinson A (1996) An overview of current cytochrome P450 technology for assessing the safety and efficacy of new materials. Toxicol Pathol 24(1):45–57
Pine MD, Hiney JK, Lee B, Les Dees W (2008) The pyrethroid pesticide esfenvalerate suppresses the afternoon rise of luteinizing hormone and delays puberty in female rats. Environ Health Perspect 116(9):1243
Dieguez C, Page MD, Scanlon MF (1988) Growth hormone neuroregulation and its alterations in disease states. Clin Endocrinol 28(1):109–143
Mula M (2008) Anticonvulsants-antidepressants pharmacokinetic drug interactions: the role of the CYP450 system in psychopharmacology. Curr Drug Metab 9(8):730–737
Tiwari AK, Deshpande SN, Rao AR, Bhatia T, Mukit SR, Shriharsh V, Lerer B, Nimagaonkar VL et al (2005) Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: I. Association of CYP1A2 gene polymorphism. Pharmacogenomics J 5(1):60–69
Daskalopoulos EP, Malliou F, Rentesi G, Marselos M, Lang MA, Konstandi M (2012) Stress is a critical player in CYP3A, CYP2C, and CYP2D regulation: role of adrenergic receptor signaling pathways. Am J Physiol Endocrinol Metab 303(1):E40–E54
Niznik HB, Tyndale RF, Sallee FR, Gonzalez FJ, Hardwick JP, Inaba T, Kalow W (1990) The dopamine transporter and cytochrome P450IID1 (debrisoquine 4-hydroxylase) in brain: resolution and identification of two distinct [3H] GBR-12935 binding proteins. Arch Biochem Biophys 276(2):424–432
Parmar D, Dayal M, Seth PK (2003) Expression of xenobiotic metabolising cytochrome P450s in brain: physiological, pharmacological and toxicological consequences. Proc Indian Natl Sci Acad B 69(6):893–916
Roberge C, Beaudet MJ, Anderson A (2004) GABA(A)/central benzodiazepine receptor and peripheral benzodiazepine receptor ligands as inducers of phenobarbital-inducible CYP2B and CYP3A. Biochem Pharmacol 68(7):1383–1389
Wojcikowski J, Golembiowska K, Daniel WA (2008) Regulation of liver cytochrome P450 by activation of brain dopaminergic system: physiological and pharmacological implications. Biochem Pharmacol 76(2):258–267
Bromek E, Haduch A, Golembiowska K, Daniel WA (2011) Cytochrome P450 mediates dopamine formation in the brain in vivo. J Neurochem 118(5):806–815
Haduch A, Bromek E, Sadakierska-Chudy A, Haduch A, Bromek E, Sadakierska-Chudy A, Wójcikowski J, Daniel WA (2013) The catalytic competence of cytochrome P450 in the synthesis of serotonin from 5-methoxytryptamine in the brain: an in vitro study. Pharmacol Res 67(1):53–59
Shahabi HN, Andersson DR, Nissbrandt H (2008) Cytochrome P450 2E1 in the substantia nigra: relevance for dopaminergic neurotransmission and free radical production. Synapse 62(5):379–388
Chandravanshi LP, Shukla RK, Sultana S, Pant AB, Khanna VK (2014) Early life arsenic exposure and brain dopaminergic alterations in rats. Int J Dev Neurosci 38:91–104
Glowinski J, Iversen LL (1966) Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem 13(8):655–669
Parmar D, Dhawan A, Seth PK (1998) Evidence for O-dealkylation of 7-pentoxyresorufin by cytochrome P450 2B1/2B2 isoenzymes in brain. Mol Cell Biochem 189(1–2):201–205
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Baldwin SJ, Bramhall JL, Ashby CA, Yue L, Murdock PR, Hood SR, Ayrton AD, Clarke SE (2006) Cytochrome P450 gene induction in rats ex vivo assessed by quantitative real-time reverse transcriptase-polymerase chain reaction (TaqMan). Drug Metab Dispos 34(6):1063–1069
Akahoshi E, Yoshimura S, Uruno S, Ishihara-Sugano M (2009) Effect of dioxins on regulation of tyrosine hydroxylase gene expression by aryl hydrocarbon receptor: a neurotoxicology study. Environ Health 8:24
Besanconon R, Reboul A, Claustrat B, Jouvet A, Belin MF, Fèvre-Montange M (1997) Tryptophan hydroxylase mRNAs analysis by RT-PCR: preliminary report on the effect of noradrenaline in the neonatal rat pineal gland. J Neurosci Res 49(6):750–758
Madziar B, Shah S, Brock M, Burke R, Lopeza-Coviella I, Nickel AC, Cakal EB, Blusztajn JK et al (2008) Nerve growth factor regulates the expression of the cholinergic locus and the high-affinity choline transporter via the Akt/PKB signaling pathway. J Neurochem 107(5):1284–1293
Stewart RR, Hoge GJ, Zigova T, Luskin MB (2002) Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABAA receptors. J Neurobiol 50(4):305–322
Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, Karmakar M, Kumari M et al (2013) Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 8(1):76–103
Mudiam MKR, Jain R, Maurya SK, Khan HA, Bandyopadhyay S, Murthy RC (2012) Low density solvent based dispersive liquid-liquid microextraction with gas chromatography-electron capture detection for the determination of cypermethrin in tissues and blood of cypermethrin treated rats. J Chromatogr B 895:65–70
Yoshinari K, Sueyoshi T, Moore R, Negishi M (2001) Nuclear receptor CAR as a regulatory factor for the sexually dimorphic induction of CYB2B1 gene by phenobarbital in rat livers. Mol Pharmacol 59(2):278–284
Sun YV, Boverhof DR, Burgoon LD, Fielden MR, Zacharewski TR (2004) Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res 32(15):4512–4523
Catinot R, Hoellinger H, Pfister A, Sonnier M, Simon MT (1989) Effects on rats of subacute intoxication with deltamethrin via an osmotic pump. Drug Chem Toxicol 12(3–4):173–196
Hedlund E, Gustafsson JA, Warner M (2001) Cytochrome P450 in the brain; a review. Curr Drug Metab 2(3):245–263
Strobel HW, Thompson CM, Antonovic L (2001) Cytochromes P450 in brain: function and significance. Curr Drug Metab 2(2):199–214
Hays LE, Carpenter CD, Petersen SL (2002) Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin during development. Environ Health Perspect 110(Suppl 3):369–376
Puga A, Tomlinson CR, Xia Y (2005) Ah receptor signals cross-talk with multiple developmental pathways. Biochem Pharmacol 69(2):199–207
Watts PM, Riedl AG, Douek DC, Edwards RJ, Boobis AR, Jenner P, Marsden CD (1998) Co-localization of P450 enzymes in the rat substantia nigra with tyrosine hydroxylase. Neuroscience 86(2):511–519
Berse B, Blusztajn JK (1997) Modulation of cholinergic locus expression by glucocorticoids and retinoic acid is cell-type specific. FEBS Lett 410(2):175–179
Barden N (1999) Regulation of corticosteroid receptor gene expression in depression and antidepressant action. J Psychiatry Neurosci 24(1):25
Hagerty T, Morgan WW, Elango N, Strong R (2001) Identification of a glucocorticoid-responsive element in the promoter region of the mouse tyrosine hydroxylase gene. J Neurochem 76(3):825–834
Uchida T, Furukawa T, Iwata S, Yanagawa Y, Fukuda A (2014) Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offsprings. Transl Psychiatry 4(3), e371
Jansson JO, Frohman LA (1987) Differential effects of neonatal and adult androgen exposure on the growth hormone secretory pattern in male rats. Endocrinology 120(4):1551–1557
Wani JH, Agrawal AK, Shapiro BH (1996) Neonatal phenobarbital-induced persistent alterations in plasma testosterone profiles and testicular function. Toxicol Appl Pharmacol 137(2):295–300
Jarukamjorn K, Sakuma T, Jaruchotikamol A, Ishino Y, Oguro M, Nemoto N (2006) Modified expression of cytochrome P450 mRNAs by growth hormone in mouse liver. Toxicology 219(1–3):97–105
Crofton KM, Reiter LW (1988) The effects of type I and II pyrethroids on motor activity and the acoustic startle response in the rat. Toxicol Sci 10(4):624–634
Stapleton G, Steel M, Richardson M, Mason JO, Rose KA, Morris RGM, Lathe R (1995) A novel cytochrome P450 expressed primarily in brain. J Biol Chem 270(50):29739–29745
Donahue CP, Kosik KS, Shors TJ (2006) Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proc Natl Acad Sci 103(15):6031–6036
Lincoln DT, El-Hifnawi E, Sinowatz F, Waters MJ (1994) Immunohistochemical localization of growth hormone receptor binding protein in the mammalian cerebellum. Ann Anat 176(5):419–427
Acknowledgments
The authors are grateful to the Director, CSIR-Indian Institute of Toxicology Research, Lucknow for his keen interest and support in carrying out the study. AS is thankful to CSIR, N. Delhi for providing Senior Research Fellowship. The financial assistance of Department of Biotechnology, N. Delhi is also gratefully acknowledged. IITR Publication No. 3141b.
Compliance with Ethical Standards
The submitted manuscript is in accordance with COPE guidelines. The authors have no potential conflicts of interest for disclosure and have no competing financial interests. The animal research has been conducted in accordance with the Declaration of Helsinki and/or with the Guide for the care and use of laboratory animals as adopted by the United States National Institute of Health. The animal experimentation was approved by the Ethical Committee of the CSIR-IITR, and all the animals were maintained in accordance to the policy laid down by the Animal Care Committee of CSIR-IITR. Informed consent received from all coauthors.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S
Homology modeling and superimposition of modeled structures over their template structures (S1a). Homology model of CYP2B1: Modeled CYP2B1 is shown in orange color. Haem group is shown in blue color. (S1b). CYP2B1 model (shown in blue) superimposed with its template structure 1suo-A (shown in Yellow color). Haem group is shown with orange color. (S2a). Homology model of CYP2E1: Modeled CYP2E1 is shown in yellow color. Haem group is shown in blue color. (S2b). CYP2E1 model (shown in green color) superimposed with its template structure 3e4e (shown in orange color). Haem group is shown in violet color. (S3a). Homology model of GABAAα1γ2 interface: Modeled GABAAα1 (shown in green color) and GABAAγ2 (shown in blue color). (S3b). GABAAα1γ2 interface (shown in blue color) superimposed over its template structure 3RHW (shown in forest green color). (S4a). Homology model of DA-D2: Model of DAD2 (shown in violet color). (S4b). Homology model of DA-D2 (shown in violet color) superimposed with its template structure 3PBL (shown in yellow color). (PPTX 2117 kb)
Rights and permissions
About this article
Cite this article
Singh, A., Mudawal, A., Maurya, P. et al. Prenatal Exposure of Cypermethrin Induces Similar Alterations in Xenobiotic-Metabolizing Cytochrome P450s and Rate-Limiting Enzymes of Neurotransmitter Synthesis in Brain Regions of Rat Offsprings During Postnatal Development. Mol Neurobiol 53, 3670–3689 (2016). https://doi.org/10.1007/s12035-015-9307-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9307-y