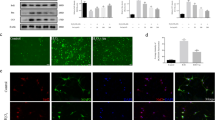
Abstract
Increased levels of ubiquitin and heat shock protein (HSP) 72 kD are often seen in spinal cord injury (SCI). However, their roles in cell injury or survival are not well known. Thus, we have investigated the possible relationship between ubiquitin and HSP expressions in relation to cell injury in healthy animals, or following nanoparticle (NP) intoxication in SCI animals. A focal SCI was inflicted on the T10–11 segments over the right dorsal horn; animals were allowed to survive from 5 to 8 h after trauma. Separate groups of rats were exposed to SiO2, Ag, or Cu NPs for 7 days and subjected to SCI on the eighth day. A marked increase in ubiquitin or HSP immunoreactive cells occurred in the T9 to T12 segments 5 h after the injury, which further extended to the T4 and L5 after 8 h of survival. At this time, a marked increase in blood–spinal cord barrier (BSCB) permeability to Evans blue and radioiodine, accompanied by an intense edema formation, was observed. Changes were further exacerbated in NP-treated traumatized rats. The most marked expressions of ubiquitin and HSP in SCI were seen in rats treated with SiO2, followed by Ag, and Cu NPs. Treatment with H-290/51 (50 mg/kg p.o., 30 to 60 min after injury) or carfilzomib (1 mg/kg, i.v., 30 to 60 min after trauma) significantly reduced the ubiquitin or HSP expressions, as well as the BSCB breakdown, the edema formation, and the cell injury in the cord both 5 and 8 h after the injury, in normal animals. However, a double dose of H-290/51 (100 mg/kg) or carfilzomib (2 mg/kg) is needed to reduce cord pathology or ubiquitin and HSP expressions in traumatized animals treated with NPs. H-290/51 showed superior beneficial effects in reducing cord pathology in SCI than carfilzomib. These observations are the first to demonstrate that (i) NP-treated traumatized animals induce a widespread BSCB leakage, edema formation, and cord pathology as well as an overexpression of ubiquitin and HSP, (ii) high doses of antioxidant compounds or proteasome inhibitors are required for neuroprotection in the NP-exposed traumatized group, and (iii) ubiquitin and HSP expressions play a key role in neuronal injury in SCI, not reported earlier.




Similar content being viewed by others
References
Campagna M, Marcias G, Angius N, Fabbri D, Noli M, Pili S, Pilia I, Avataneo G et al (2014) 0277 Environmental exposure to nanoparticles in Sardinia, Italy: a pilot study of residential exposure nearby an industrial area and a military shooting range. Occup Environ Med 71(Suppl 1):A99. doi:10.1136/oemed-2014-102362.310
CPT Korami Dembele. Determining Nanoparticle Inhalation Exposure in the Prosthetics Laboratory at Walter Reed National Military Medical Center, Master Thesis, 2013, UNIFORMED SERVICES UNIVERSITY, SCHOOL OF MEDICINE GRADUATE PROGRAMS Graduate Education Office (A 1045), 4301 Jones Bridge Road, Bethesda, MD 20814
Unintended Nanoparticles: The most dangerous yet? Military Problems and Nanotechnology Solutions. http://www.nanomagazine.co.uk/index.php?option=com_content&view=article&id=200:article-unintended-nanoparticles-the-most-dangerous-yet&catid=56&Itemid=151. Accessed 15 Jul 2014
Potential Environmental Pollution and Health Hazards Resulting from Possible Military Uses of Nanotechnology with Implications for Research Priorities Helpful to Prevent and/or Reduce Such Pollution and Hazards. Millennium Project of the American Council for the United Nations University for the Army Environmental Policy Institute, February 2005, http://www.prospective-foresight.com/IMG/doc/MP_nanotech_report.doc. Accessed on 23 Jan 2015
Fritz HA, Lysack C, Luborsky MR, Messinger SD (2014) Long-term community reintegration: concepts, outcomes and dilemmas in the case of a military service member with a spinal cord injury. Disabil Rehabil Oct 1:1–7
Rivera JC, Anderson ER, Jenne JW, Topp RF (2014) Spine-related disability following combat injury. J Surg Orthop Adv Fall 23(3):136–139
Schoenfeld AJ, Laughlin MD, McCriskin BJ, Bader JO, Waterman BR, Belmont PJ Jr (2013) Spinal injuries in United States military personnel deployed to Iraq and Afghanistan: an epidemiological investigation involving 7877 combat casualties from 2005 to 2009. Spine 38(20):1770–1778. doi:10.1097/BRS.0b013e31829ef226
Schoenfeld AJ, Newcomb RL, Pallis MP, Cleveland AW 3rd, Serrano JA, Bader JO, Waterman BR, Belmont PJ Jr (2013) Characterization of spinal injuries sustained by American service members killed in Iraq and Afghanistan: a study of 2,089 instances of spine trauma. J Trauma Acute Care Surg 74(4):1112–1118. doi:10.1097/TA.0b013e31828273be
Blair JA, Patzkowski JC, Schoenfeld AJ, Cross Rivera JD, Grenier ES, Lehman RA Jr, Hsu JR, Skeletal Trauma Research Consortium (STReC) (2012) Spinal column injuries among Americans in the global war on terrorism. J Bone Joint Surg Am 94(18):e135(1–9)
Karr JE, Areshenkoff CN, Duggan EC, Garcia-Barrera MA (2014) Blast-related mild traumatic brain injury: a Bayesian random-effects meta-analysis on the cognitive outcomes of concussion among military personnel. Neuropsychol Rev 24(4):428–444. doi:10.1007/s11065-014-9271-8
Bell RS, Vo AH, Neal CJ, Tigno J, Roberts R, Mossop C, Dunne JR, Armonda RA (2009) Military traumatic brain and spinal column injury: a 5-year study of the impact blast and other military grade weaponry on the central nervous system. J Trauma 66(4 Suppl):S104–S111. doi:10.1097/TA.0b013e31819d88c8
Huang H, Sun T, Chen L, Moviglia G, Chernykh E, Wild KV, Deda H, Kang KS et al (2014) Consensus of clinical neurorestorative progresses in patients with complete chronic spinal cord injury. Cell Transplant 23(Suppl 1):5–17. doi:10.3727/096368914X684952
Sharma HS (2008) New perspectives for the treatment options in spinal cord injury. Expert Opin Pharmacother 9(16):2773–2800. doi:10.1517/14656566.9.16.2773
Sharma HS (2012) New Perspectives of Central Nervous System Injury and Neuroprotection. International Review of Neurobiology, vol 102. Academic, Oxford, pp 1–336
Sharma HS, Westman J (2004) The blood-spinal cord and brain barriers in health and disease. Academic, San Diego, pp 1–617
Stålberg E, Sharma HS, Olsson Y (1998) Spinal cord monitoring. Basic principles, regeneration, pathophysiology and clinical aspects. Springer, Wien New York, pp 1–527
Sharma HS (2005) Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des 11(11):1353–1389
Sharma HS (2004) Pathophysiology of the blood-spinal cord barrier in traumatic injury. In: Sharma HS, Westman J (eds) The blood-spinal cord and brain barriers in health and disease. Academic, San Diego, pp 437–518
Sharma HS, Olsson Y (1990) Edema formation and cellular alterations following spinal cord injury in rat and their modification with p-chlorophenylalanine. Acta Neuropathol (Berlin) 79:604–610
Sharma HS, Olsson Y, Pearsson S, Nyberg F (1995) Trauma induced opening of the blood-spinal cord barrier is reduced by indomethacin, an inhibitor of prostaglandin synthesis. Experimental observations in the rat using 131I-sodium, Evans blue and lanthanum as tracers. Res Neurol & Neurosci 7:207–215
Sharma HS (2011) Early microvascular reactions and blood-spinal cord barrier disruption are instrumental in pathophysiology of spinal cord injury and repair: novel therapeutic strategies including nanowired drug delivery to enhance neuroprotection. J Neural Transm Jan 118(1):155–176
Sharma HS, Gordh T, Wiklund L, Mohanty S, Sjöquist PO (2006) Spinal cord injury induced heat shock protein expression is reduced by an antioxidant compound H-290/51. An experimental study using light and electron microscopy in the rat. J Neural Transm 113(4):521–536
Fagoe ND, van Heest J, Verhaagen J (2014) Spinal cord injury and the neuron-intrinsic regeneration-associated gene program. Neuromolecular Med 16(4):799–813. doi:10.1007/s12017-014-8329-3
Ciechanover A (2013) Intracellular protein degradation: from a vague idea through the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Bioorg Med Chem 21(12):3400–3410. doi:10.1016/j.bmc.2013.01.056
Jankowska E, Stoj J, Karpowicz P, Osmulski PA, Gaczynska M (2013) The proteasome in health and disease. Curr Pharm Des 19(6):1010–1028
Braten O, Shabek N, Kravtsova-Ivantsiv Y, Ciechanover A (2012) Generation of free ubiquitin chains is up-regulated in stress and facilitated by the HECT domain ubiquitin ligases UFD4 and HUL5. Biochem J 444(3):611–617. doi:10.1042/BJ20111840
Weissman AM, Shabek N, Ciechanover A (2011) The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat Rev Mol Cell Biol 12(9):605–620. doi:10.1038/nrm3173, Review. Erratum in: Nat Rev Mol Cell Biol. Oct;12(10):686
Saito R, Kaneko M, Kitamura Y, Takata K, Kawada K, Okuma Y, Nomura Y (2014) Effects of oxidative stress on the solubility of HRD1, a ubiquitin ligase implicated in Alzheimer’s disease. PLoS One 9(5), e94576. doi:10.1371/journal.pone.0094576, eCollection 2014
Jansen AH, Reits EA, Hol EM (2014) The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci 7:73. doi:10.3389/fnmol.2014.00073
Wang H, Saunders AJ (2014) The role of ubiquitin-proteasome in the metabolism of amyloid precursor protein (APP): implications for novel therapeutic strategies for Alzheimer’s disease. Discov Med 18(97):41–50
Atkin G, Paulson H (2014) Ubiquitin pathways in neurodegenerative disease. Front Mol Neurosci 7:63. doi:10.3389/fnmol.2014.00063
Arnaoutakis GJ, George TJ, Wang KK, Wilson MA, Allen JG, Robinson CW, Haggerty KA, Weiss ES et al (2011) Serum levels of neuron-specific ubiquitin carboxyl-terminal esterase-L1 predict brain injury in a canine model of hypothermic circulatory arrest. J Thorac Cardiovasc Surg 142(4):902–910.e1. doi:10.1016/j.jtcvs.2011.06.027
Puvenna V, Brennan C, Shaw G, Yang C, Marchi N, Bazarian JJ, Merchant-Borna K, Janigro D (2014) Significance of ubiquitin carboxy-terminal hydrolase L1 elevations in athletes after sub-concussive head hits. PLoS One 9(5), e96296. doi:10.1371/journal.pone.0096296
Benter IF, Abul HT, Al-Khaledi G, Renno WM, Canatan H, Akhtar S (2011) Inhibition of Ras-GTPase farnesylation and the ubiquitin-proteasome system or treatment with angiotensin-(1–7) attenuates spinal cord injury-induced cardiac dysfunction. J Neurotrauma 28(7):1271–1279. doi:10.1089/neu.2010.1682
Yang W, Sheng H, Thompson JW, Zhao S, Wang L, Miao P, Liu X, Moseley MA et al (2014) Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke 45(4):1115–1122. doi:10.1161/STROKEAHA.113.004315
Noor NM, Møllgård K, Wheaton BJ, Steer DL, Truettner JS, Dziegielewska KM, Dietrich WD, Smith AI et al (2013) Expression and cellular distribution of ubiquitin in response to injury in the developing spinal cord of Monodelphis domestica. PLoS One 8(4), e62120. doi:10.1371/journal.pone.0062120. Print 2013
Benter IF, Abul HT, Al-Khaledi G, Renno WM, Canatan H, Akhtar S (2011) Inhibition of Ras-GTPase farnesylation and the ubiquitin-proteasome system or treatment with angiotensin-(1–7) attenuates spinal cord injury-induced cardiac dysfunction. J Neurotrauma 28(7):1271–1279. doi:10.1089/neu.2010.1682
Yamauchi T, Sakurai M, Abe K, Matsumiya G, Sawa Y (2008) Ubiquitin-mediated stress response in the spinal cord after transient ischemia. Stroke 39(6):1883–1889. doi:10.1161/STROKEAHA.106.455832
Ossipov MH, Bazov I, Gardell LR, Kowal J, Yakovleva T, Usynin I, Ekström TJ, Porreca F et al (2007) Control of chronic pain by the ubiquitin proteasome system in the spinal cord. J Neurosci 27(31):8226–8237
Derouiche F, Bôle-Feysot C, Naïmi D, Coëffier M (2014) Hyperhomocysteinemia-induced oxidative stress differentially alters proteasome composition and activities in heart and aorta. Biochem Biophys Res Commun 452(3):740–745. doi:10.1016/j.bbrc.2014.08.141
Fang NN, Chan GT, Zhu M, Comyn SA, Persaud A, Deshaies RJ, Rotin D, Gsponer J et al (2014) sp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress. Nat Cell Biol. doi:10.1038/ncb3054
Lantéri-Minet M, Desmeules JA, Menétrey D (1993) Opposite effects of axon damage on heat shock proteins (hsp 70) and ubiquitin contents in motor neurons of neuropathic rats. Neurosci Lett 153(1):49–52
Nakasone N, Nakamura YS, Higaki K, Oumi N, Ohno K, Ninomiya H (2014) Endoplasmic reticulum-associated degradation of Niemann-Pick C1: evidence for the role of heat shock proteins and identification of lysine residues that accept ubiquitin. J Biol Chem 289(28):19714–19725. doi:10.1074/jbc.M114.549915
Mustafa A, Sharma HS, Olsson Y, Gordh T, Thóren P, Sjöquist PO, Roos P, Adem A et al (1995) Vascular permeability to growth hormone in the rat central nervous system after focal spinal cord injury. Influence of a new anti-oxidant H 290/51 and age. Neurosci Res 23(2):185–194
Sharma HS, Sjöquist PO (2002) A new antioxidant compound H-290/51 modulates glutamate and GABA immunoreactivity in the rat spinal cord following trauma. Amino Acids 23(1–3):261–272
Sharma HS, Sjöquist PO, Mohanty S, Wiklund L (2006) Post-injury treatment with a new antioxidant compound H-290/51 attenuates spinal cord trauma-induced c-fos expression, motor dysfunction, edema formation, and cell injury in the rat. Acta Neurochir Suppl 96:322–328
Sharma HS, Sjöquist PO, Alm P (2003) A new antioxidant compound H-290151 attenuates spinal cord injury induced expression of constitutive and inducible isoforms of nitric oxide synthase and edema formation in the rat. Acta Neurochir Suppl 86:415–420
Calzolai L, Franchini F, Gilliland D, Rossi F (2010) Protein–nanoparticle interaction: identification of the ubiquitin–gold nanoparticle interaction site. Nano Lett 10(8):3101–3105. doi:10.1021/nl101746v
Mangini V, Dell’Aglio M, De Stradis A, De Giacomo A, De Pascale O, Natile G, Arnesano F (2014) Amyloid transition of ubiquitin on silver nanoparticles produced by pulsed laser ablation in liquid as a function of stabilizer and single-point mutations. Chemistry 20(34):10745–10751. doi:10.1002/chem.201402934
Li YJ, Perkins AL, Su Y, Ma Y, Colson L, Horne DA, Chen Y (2012) Gold nanoparticles as a platform for creating a multivalent poly-SUMO chain inhibitor that also augments ionizing radiation. Proc Natl Acad Sci U S A 109(11):4092–4097. doi:10.1073/pnas.1109131109
Brancolini G, Kokh DB, Calzolai L, Wade RC, Corni S (2012) Docking of ubiquitin to gold nanoparticles. ACS Nano 6(11):9863–9878. doi:10.1021/nn303444b
Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 8th Edn, 2011, The National Academic Press, Washington DC, http://www.nap.edu
Sharma HS, Sharma A (2012) Rodent spinal cord injury model and application of neurotrophic factors for neuroprotection. Methods Mol Biol 846:393–415. doi:10.1007/978-1-61779-536-7_33
Sharma HS, Muresanu DF, Patnaik R, Sharma A (2013) Exacerbation of brain pathology after partial restraint in hypertensive rats following SiO2 nanoparticles exposure at high ambient temperature. Mol Neurobiol 48(2):368–379. doi:10.1007/s12035-013-8502-y
Sharma A, Muresanu DF, Patnaik R, Sharma HS (2013) Size- and age-dependent neurotoxicity of engineered metal nanoparticles in rats. Mol Neurobiol 48(2):386–396. doi:10.1007/s12035-013-8500-0
Sharma HS, Ali SF, Tian ZR, Hussain SM, Schlager JJ, Sjöquist PO, Sharma A, Muresanu DF (2009) Chronic treatment with nanoparticles exacerbate hyperthermia induced blood–brain barrier breakdown, cognitive dysfunction and brain pathology in the rat. Neuroprotective effects of nanowired-antioxidant compound H-290/51. J Nanosci Nanotechnol 9(8):5073–5090
Sharma HS, Ali SF, Hussain SM, Schlager JJ, Sharma A (2009) Influence of engineered nanoparticles from metals on the blood–brain barrier permeability, cerebral blood flow, brain edema and neurotoxicity. An experimental study in the rat and mice using biochemical and morphological approaches. J Nanosci Nanotechnol 9(8):5055–5072
Highsmith KN, Chen SE, Horowitz S (2014) Carfilzomib and pomalidomide: recent advances in the treatment of multiple myeloma. Pharmacotherapy 34(9):927–940. doi:10.1002/phar.1463
Stintzing S, Lenz HJ (2014) Molecular pathways: turning proteasomal protein degradation into a unique treatment approach. Clin Cancer Res 20(12):3064–3070. doi:10.1158/1078-0432.CCR-13-3175
Johnson D (2015) The ubiquitin-proteasome system: opportunities for therapeutic intervention in solid tumors. Endocr Relat Cancer 22(1):T1–T17
Elliott KA, Jasper HH (1949) Measurement of experimentally induced brain swelling and shrinkage. Am J Physiol 157(1):122–129
Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A (1999) A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods 93(2):149–162
Kato S, Hirano A, Kato M, Herz F, Ohama E (1993) Comparative study on the expression of stress-response protein (srp) 72, srp 27, alpha B-crystallin and ubiquitin in brain tumours. An immunohistochemical investigation. Neuropathol Appl Neurobiol 19(5):436–442
Chu CT, Caruso JL, Cummings TJ, Ervin J, Rosenberg C, Hulette CM (2000) Ubiquitin immunochemistry as a diagnostic aid for community pathologists evaluating patients who have dementia. Mod Pathol 13(4):420–426
Sharma HS, Olsson Y, Westman J (1995) A serotonin synthesis inhibitor, p-chlorophenylalanine reduces the heat shock protein response following trauma to the spinal cord: an immunohistochemical and ultrastructural study in the rat. Neurosci Res 21(3):241–249
Sharma HS, Westman J (1997) Prostaglandins modulate constitutive isoform of heat shock protein (72 kD) response following trauma to the rat spinal cord. Acta Neurochir Suppl 70:134–137
Kiyatkin EA, Sharma HS (2011) Expression of heat shock protein (HSP 72 kDa) during acute methamphetamine intoxication depends on brain hyperthermia: neurotoxicity or neuroprotection? J Neural Transm 118(1):47–60. doi:10.1007/s00702-010-0477-5
Menon PK, Muresanu DF, Sharma A, Mössler H, Sharma HS (2012) Cerebrolysin, a mixture of neurotrophic factors induces marked neuroprotection in spinal cord injury following intoxication of engineered nanoparticles from metals. CNS Neurol Disord Drug Targets 11(1):40–49
Sharma HS, Sharma A (2012) Nanowired drug delivery for neuroprotection in central nervous system injuries: modulation by environmental temperature, intoxication of nanoparticles, and comorbidity factors. Wiley Interdiscip Rev Nanomed Nanobiotechnol 4(2):184–203
Sharma HS, Sharma A (2013) New perspectives of nanoneuroprotection, nanoneuropharmacology and nanoneurotoxicity: modulatory role of amino acid neurotransmitters, stress, trauma, and co-morbidity factors in nanomedicine. Amino Acids 45(5):1055–1071. doi:10.1007/s00726-013-1584-z
Sharma HS, Patnaik R, Sharma A, Sjöquist PO, Lafuente JV (2009) Silicon dioxide nanoparticles (SiO2, 40–50 nm) exacerbate pathophysiology of traumatic spinal cord injury and deteriorate functional outcome in the rat. An experimental study using pharmacological and morphological approaches. J Nanosci Nanotechnol 9(8):4970–4980
Sharma HS, Muresanu DF, Sharma A, Patnaik R, Lafuente JV (2009) Nanoparticles influence pathophysiology of spinal cord injury and repair. Prog Brain Res 180:154–180. doi:10.1016/S0079-6123(08)80009-X
Xin L, Wang J, Wu Y, Guo S, Tong J (2014) Increased oxidative stress and activated heat shock proteins in human cell lines by silver nanoparticles. Hum Exp Toxicol. Jun 30.
Huerta-García E, Pérez-Arizti JA, Márquez-Ramírez SG, Delgado-Buenrostro NL, Chirino YI, Iglesias GG, López-Marure R (2014) Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic Biol Med 73:84–94. doi:10.1016/j.freeradbiomed.2014.04.026
Sharma HS, Sjöquist P-O, Westman J (2001) Pathophysiology of the blood-spinal cord barrier in spinal cord injury. Influence of a new antioxidant compound H-290/51. In: Kobiler D, Lustig S, Shapra S (eds) Blood–brain barrier.Drug delivery and brain pathology. Kluwer Academic/Plenum Publishers, New York, pp 401–416
Acknowledgments
The Authors (HS/AS) are deeply obliged for the encouragement of our Ubiquitin work by Nobel Laureate Aaron Ciechanover during 3rd GTC Congress, Las Vegas, NV, Feb 24–26, 2013. This investigation is supported by grants from the Air Force Office of Scientific Research (EOARD, London, UK), and Air Force Material Command, USAF, under grant number FA8655-05-1-3065; Swedish Medical Research Council (Nr 2710-HSS), Swedish Strategic Research Foundation, Stockholm, Sweden; Göran Gustafsson Foundation, Stockholm, Sweden (HSS), Astra Zeneca, Mölndal, Sweden (HSS/AS), The University Grants Commission, New Delhi, India (HSS/AS), Ministry of Science & Technology, Govt. of India & Govt. of Sweden (HSS/AS), Indian Medical Research Council, New Delhi, India (HSS/AS); India-EU Research Co-operation Program (RP/AS/HSS) and IT 794/13 (JVL), Government of Basque Country and UFI 11/32 (JVL); University of Basque Country, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, H.S., Muresanu, D.F., Lafuente, J.V. et al. Nanoparticles Exacerbate Both Ubiquitin and Heat Shock Protein Expressions in Spinal Cord Injury: Neuroprotective Effects of the Proteasome Inhibitor Carfilzomib and the Antioxidant Compound H-290/51. Mol Neurobiol 52, 882–898 (2015). https://doi.org/10.1007/s12035-015-9297-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9297-9