Abstract
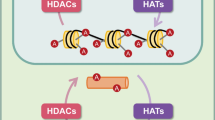
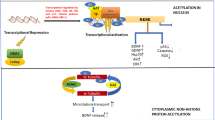
Small molecule histone deacetylase inhibitors (HDIs) hold much promise as pharmacological modifiers of the epigenetic status of the central nervous system (CNS), given their ability to cross the blood-brain barrier. This is particularly relevant given the lack of disease-modifying therapies for many neurodegenerative diseases and that epigenetic perturbations are increasingly recognised as playing a key role in their pathophysiology. In particular, emerging evidence in recent years has shown that epigenetic dysregulation may contribute to dopaminergic neuronal death in Parkinson’s disease. As a result, a number of pan-HDIs have been explored as potential neuroprotective agents for dopaminergic neurons. However, it is not known if the neuroprotective effects of pan-histone deacetylase (HDAC) inhibition are a general phenomenon or if these effects require inhibition of specific classes of HDACs. Here, we examine the ability of class-specific HDIs to promote neurite growth in a variety of cellular contexts. We find that MC1568, a class IIa-specific HDI, promotes neurite growth and arbourisation and protects neurite arbours against neurotoxic insult. Furthermore, we show that class IIa-specific HDAC inhibition results in activation of the canonical Smad signalling pathway, which is known to promote the survival and growth of midbrain dopaminergic neurons. These results demonstrate the potential of class IIa-specific HDIs as regulators of neuronal structure and suggest they should be examined in animal models of Parkinson’s disease as the next stage in rationalising their use as a potential therapy for this disorder.





Similar content being viewed by others
References
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066. doi:10.1016/S0140-6736(09)60492-X
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. doi:10.1136/jnnp.2007.131045
Bethlem J, Den Hartog Jager WA (1960) The incidence and characteristics of Lewy bodies in idiopathic paralysis agitans (Parkinson’s disease). J Neurol Neurosurg Psychiatry 23:74–80
Jellinger KA (1991) Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14(3):153–197
Jellinger KA (2012) Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord 27(1):8–30. doi:10.1002/mds.23795
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222. doi:10.1146/annurev-pathol-011110-130242
Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK (2003) Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur J Neurosci 18(5):1175–1188
Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK (2000) Axonal sprouting following lesions of the rat substantia nigra. Neuroscience 97(1):99–112
Bedard C, Wallman MJ, Pourcher E, Gould PV, Parent A, Parent M (2011) Serotonin and dopamine striatal innervation in Parkinson’s disease and Huntington’s chorea. Parkinsonism Relat Disord 17(8):593–598. doi:10.1016/j.parkreldis.2011.05.012
Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO 3rd (2000) Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med 133(5):338–347. doi:10.7326/0003-4819-133-5-200009050-00009
Kaufmann H, Goldstein DS (2013) Autonomic dysfunction in Parkinson disease. Handb Clin Neurol 117:259–278. doi:10.1016/B978-0-444-53491-0.00021-3
Goldstein DS, Holmes C, Cannon RO 3rd, Eisenhofer G, Kopin IJ (1997) Sympathetic cardioneuropathy in dysautonomias. N Engl J Med 336(10):696–702. doi:10.1056/NEJM199703063361004
Cascini LG, Cuccurullo V, Restuccia A, Tamburrini O, Rotondo A, Mansi L (2013) Neurological applications for myocardial MIBG scintigraphy. Nucl Med Rev Cent East Eur 16(1):35–41. doi:10.5603/NMR.2013.0007
Toulouse A, Sullivan AM (2008) Progress in Parkinson’s disease—where do we stand? Prog Neurobiol 85(4):376–392. doi:10.1016/j.pneurobio.2008.05.003
Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9(5):589–595. doi:10.1038/nm850
Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59(3):459–466. doi:10.1002/ana.20737
Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS (2005) Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol 57(2):298–302. doi:10.1002/ana.20374
Sullivan AM, Toulouse A (2011) Neurotrophic factors for the treatment of Parkinson’s disease. Cytokine Growth Factor Rev 22(3):157–165. doi:10.1016/j.cytogfr.2011.05.001
Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B (2005) Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg 102(2):216–222. doi:10.3171/jns.2005.102.2.0216
Harrison IF, Dexter DT (2013) Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson’s disease? Pharmacol Ther 140(1):34–52. doi:10.1016/j.pharmthera.2013.05.010
Allfrey VG, Faulkner R, Mirsky AE (1964) Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci U S A 51:786–794
Serrano L, Vazquez BN, Tischfield J (2013) Chromatin structure, pluripotency and differentiation. Exp Biol Med (Maywood) 238(3):259–270. doi:10.1177/1535370213480718
D’Arcy S, Luger K (2011) Understanding histone acetyltransferase Rtt109 structure and function: how many chaperones does it take? Curr Opin Struct Biol 21(6):728–734. doi:10.1016/j.sbi.2011.09.005
Gallwitz D, Sures I (1972) Histone acetylation. Purification and properties of three histone-specific acetyltransferases from rat thymus nuclei. Biochim Biophys Acta 263(2):315–328
Fujimoto D (1972) Specificities of histone deacetylases from several animal and plant tissues. J Biochem 72(5):1269–1271
Murakami Y (2013) Histone deacetylases govern heterochromatin in every phase. EMBO J. doi:10.1038/emboj.2013.154
Konsoula Z, Barile FA (2012) Epigenetic histone acetylation and deacetylation mechanisms in experimental models of neurodegenerative disorders. J Pharmacol Toxicol Methods 66(3):215–220. doi:10.1016/j.vascn.2012.08.001
Bjerling P, Silverstein RA, Thon G, Caudy A, Grewal S, Ekwall K (2002) Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity. Mol Cell Biol 22(7):2170–2181. doi:10.1128/MCB.22.7.2170-2181.2002
Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E (2002) Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell 9(1):45–57. doi:10.1016/S1097-2765(01)00429-4
De Ruijter A, Van Gennip A, Caron H, Kemp S, van Kuilenburg A (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370:737–749. doi:10.1042/BJ20021321
Abel T, Zukin RS (2008) Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol 8(1):57–64. doi:10.1016/j.coph.2007.12.002
Chuang D-M, Leng Y, Marinova Z, Kim H-J, Chiu C-T (2009) Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci 32(11):591–601. doi:10.1016/j.tins.2009.06.002
Hahnen E, Hauke J, Tränkle C, Eyüpoglu IY, Wirth B, Blümcke I (2008) Histone deacetylase inhibitors: possible implications for neurodegenerative disorders. 17(2):169–184. doi:10.1517/13543784.17.2.169
Mai A, Rotili D, Valente S, Kazantsev AG (2009) Histone deacetylase inhibitors and neurodegenerative disorders: holding the promise. Curr Pharm Des 15(34):3940–3957. doi:10.2174/138161209789649349
Peedicayil J (2014) Epigenetic Drugs in cognitive disorders. Curr Pharm Des 20(11):1840–1846. doi:10.2174/13816128113199990526
Xu Z, Li H, Jin P (2012) Epigenetics-based therapeutics for neurodegenerative disorders. Curr Transl Geriatr Exp Gerontol Rep 1(4):229–236. doi:10.1007/s13670-012-0027-0
Chen PS, Wang C-C, Bortner CD, Peng G-S, Wu X, Pang H, Lu R-B, Gean P-W, Chuang D-M, Hong J-S (2007) Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 149(1):203–212. doi:10.1016/j.neuroscience.2007.06.053
Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, Moroni F, Chiarugi A (2006) Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol 70(6):1876–1884. doi:10.1124/mol.106.027912
Petri S, Kiaei M, Kipiani K, Chen J, Calingasan NY, Crow JP, Beal MF (2006) Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 22(1):40–49. doi:10.1016/j.nbd.2005.09.013
Kim HJ, Rowe M, Ren M, Hong J-S, Chen P-S, Chuang D-M (2007) Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 321(3):892–901. doi:10.1124/jpet.107.120188
Leng Y, Marinova Z, Reis-Fernandes MA, Nau H, Chuang D-M (2010) Potent neuroprotective effects of novel structural derivatives of valproic acid: potential roles of HDAC inhibition and HSP70 induction. Neurosci Lett 476(3):127–132. doi:10.1016/j.neulet.2010.04.013
Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, Leeds P, Chuang DM (2009) Valproic acid induces functional heat‐shock protein 70 via class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J Neurochem 111(4):976–987. doi:10.1111/j.1471-4159.2009.06385.x
Lv L, Sun Y, Han X, C-c X, Tang Y-P, Dong Q (2011) Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain Res 1396:60–68. doi:10.1016/j.brainres.2011.03.040
Chen PS, Peng G, Li G, Yang S, Wu X, Wang C, Wilson B, Lu R, Gean P-W, Chuang D (2006) Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry 11(12):1116–1125. doi:10.1038/sj.mp.4001893
Peng G-S, Li G, Tzeng N-S, Chen P-S, Chuang D-M, Hsu Y-D, Yang S, Hong J-S (2005) Valproate pretreatment protects dopaminergic neurons from LPS-induced neurotoxicity in rat primary midbrain cultures: role of microglia. Mol Brain Res 134(1):162–169. doi:10.1016/j.molbrainres.2004.10.021
Leng Y, Liang M-H, Ren M, Marinova Z, Leeds P, Chuang D-M (2008) Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci 28(10):2576–2588. doi:10.1523/JNEUROSCI.5467-07.2008
Oliveira JM, Chen S, Almeida S, Riley R, Gonçalves J, Oliveira CR, Hayden MR, Nicholls DG, Ellerby LM, Rego AC (2006) Mitochondrial-dependent Ca2+ handling in Huntington’s disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci 26(43):11174–11186. doi:10.1523/JNEUROSCI.3004-06.2006
Langley B, D’Annibale MA, Suh K, Ayoub I, Tolhurst A, Bastan B, Yang L, Ko B, Fisher M, Cho S (2008) Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21waf1/cip1 in cell cycle-independent neuroprotection. J Neurosci 28(1):163–176. doi:10.1523/JNEUROSCI.3200-07.2008
Ryu H, Lee J, Olofsson BA, Mwidau A, Deodoglu A, Escudero M, Flemington E, Azizkhan-Clifford J, Ferrante RJ, Ratan RR (2003) Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci 100(7):4281–4286. doi:10.1073/pnas.0737363100
Schroeder FA, Lin CL, Crusio WE, Akbarian S (2007) Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 62(1):55–64. doi:10.1016/j.biopsych.2006.06.036
van Heesbeen HJ, Mesman S, Veenvliet JV, Smidt MP (2013) Epigenetic mechanisms in the development and maintenance of dopaminergic neurons. Development 140(6):1159–1169. doi:10.1242/dev.089359
Zhu M, Li WW, Lu CZ (2014) Histone deacetylase inhibitors prevent mitochondrial fragmentation and elicit early neuroprotection against MPP+. CNS Neurosci Ther 20(4):308–316. doi:10.1111/cns.12217
Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang C-C, Kinyamu H, Lu N, Gao X, Leng Y (2008) Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol 11(08):1123–1134. doi:10.1017/S1461145708009024
Kidd SK, Schneider JS (2010) Protection of dopaminergic cells from MPP + -mediated toxicity by histone deacetylase inhibition. Brain Res 1354:172–178. doi:10.1016/j.brainres.2010.07.041
Gardian G, Yang L, Cleren C, Calingasan NY, Klivenyi P, Beal MF (2004) Neuroprotective effects of phenylbutyrate against MPTP neurotoxicity. Neruomol Med 5(3):235–241. doi:10.1385/NMM:5:3:235
Kidd S, Schneider J (2011) Protective effects of valproic acid on the nigrostriatal dopamine system in a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience 194:189–194. doi:10.1016/j.neuroscience.2011.08.010
Laurent RS, O’Brien L, Ahmad S (2013) Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience 246:382–390. doi:10.1016/j.neuroscience.2013.04.037
Kontopoulos E, Parvin JD, Feany MB (2006) α-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet 15(20):3012–3023. doi:10.1093/hmg/ddl243
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet J-C, McLean PJ (2007) Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317(5837):516–519. doi:10.1126/science.1143780
Fischer A, Sananbenesi F, Mungenast A, Tsai L-H (2010) Targeting the correct HDAC (s) to treat cognitive disorders. Trends Pharmacol Sci 31(12):605–617. doi:10.1016/j.tips.2010.09.003
Dietz KC, Casaccia P (2010) HDAC inhibitors and neurodegeneration: at the edge between protection and damage. Pharmacol Res 62(1):11–17. doi:10.1016/j.phrs.2010.01.011
Hegarty SV, Sullivan AM, O’Keeffe GW (2013) BMP2 and GDF5 induce neuronal differentiation through a Smad dependant pathway in a model of human midbrain dopaminergic neurons. Mol Cell Neurosci 56:263–271. doi:10.1016/j.mcn.2013.06.006
Nolan AM, Nolan YM, O’Keeffe GW (2011) IL-1β inhibits axonal growth of developing sympathetic neurons. Mol Cell Neurosci 48(2):142–150. doi:10.1016/j.mcn.2011.07.003
Collins L, O’Keeffe G, Long-Smith C, Wyatt S, Sullivan A, Toulouse A, Nolan Y (2013) Mitogen-activated protein kinase phosphatase (MKP)-1 as a neuroprotective agent: promotion of the morphological development of midbrain dopaminergic neurons. Neuromol Med 15(2):435–446. doi:10.1007/s12017-013-8230-5
Gutierrez H, Davies AM (2007) A fast and accurate procedure for deriving the Sholl profile in quantitative studies of neuronal morphology. J Neurosci Methods 163(1):24–30. doi:10.1016/j.jneumeth.2007.02.002
Crampton SJ, Collins LM, Toulouse A, Nolan YM, O’Keeffe GW (2012) Exposure of foetal neural progenitor cells to IL-1β impairs their proliferation and alters their differentiation—a role for maternal inflammation? J Neurochem 120(6):964–973. doi:10.1111/j.1471-4159.2011.07634.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Xie H-r HL-S, Li G-Y (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J (Engl) 123(8):1086–1092
Hegarty SV, Sullivan AM, O’Keeffe GW (2013) BMP2 and GDF5 induce neuronal differentiation through a Smad dependant pathway in a model of human midbrain dopaminergic neurons. Mol Cell Neurosci. doi:10.1016/j.mcn.2013.06.006
Hegarty SV, Collins LM, Gavin AM, Roche SL, Wyatt SL, Sullivan AM, O’Keeffe GW (2014) Canonical BMP-Smad signalling promotes neurite growth in rat midbrain dopaminergic neurons. Neruomol Med. doi:10.1007/s12017-014-8299-5
Yuan P-X, Huang L-D, Jiang Y-M, Gutkind JS, Manji HK, Chen G (2001) The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem 276(34):31674–31683. doi:10.1074/jbc.M104309200
Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G (2004) Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci 24(29):6590–6599. doi:10.1523/JNEUROSCI.5747-03.2004
Rößler R, Boddeke E, Copray S (2010) Differentiation of non-mesencephalic neural stem cells towards dopaminergic neurons. Neuroscience 170(2):417–428. doi:10.1016/j.neuroscience.2010.07.023
Salminen A, Tapiola T, Korhonen P, Suuronen T (1998) Neuronal apoptosis induced by histone deacetylase inhibitors. Mol Brain Res 61(1–2):203–206. doi:10.1016/S0169-328X(98)00210-1
Wang Y, Wang X, Liu L, Wang X (2009) HDAC inhibitor trichostatin A-inhibited survival of dopaminergic neuronal cells. Neurosci Lett 467(3):212–216. doi:10.1016/j.neulet.2009.10.037
Chen SH, Wu HM, Ossola B, Schendzielorz N, Wilson BC, Chu CH, Chen SL, Wang Q, Zhang D, Qian L, Li X, Hong JS, Lu RB (2012) Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, protects dopaminergic neurons from neurotoxin-induced damage. Br J Pharmacol 165(2):494–505. doi:10.1111/j.1476-5381.2011.01575.x
Ramsay RR, Krueger MJ, Youngster SK, Gluck MR, Casida JE, Singer TP (1991) Interaction of 1‐methyl‐4‐phenylpyridinium ion (MPP+) and its analogs with the rotenone/piericidin binding site of NADH dehydrogenase. J Neurochem 56(4):1184–1190
Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S (2010) HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ 17(9):1392–1408. doi:10.1038/cdd.2009.216
Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y (2007) New nomenclature for chromatin-modifying enzymes. Cell 131(4):633–636. doi:10.1016/j.cell.2007.10.039
Bugyei-Twum A, Advani A, Advani SL, Zhang Y, Thai K, Kelly DJ, Connelly KA (2014) High glucose induces Smad activation via the transcriptional coregulator p300 and contributes to cardiac fibrosis and hypertrophy. Cardiovasc Diabetol 13:89. doi:10.1186/1475-2840-13-89
Yuan H, Reddy MA, Sun G, Lanting L, Wang M, Kato M, Natarajan R (2013) Involvement of p300/CBP and epigenetic histone acetylation in TGF-beta1-mediated gene transcription in mesangial cells. Am J Physiol Renal Physiol 304(5):F601–F613. doi:10.1152/ajprenal.00523.2012
Takahashi-Fujigasaki J, Fujigasaki H (2006) Histone deacetylase (HDAC) 4 involvement in both Lewy and Marinesco bodies. Neuropathol Appl Neurobiol 32(5):562–566. doi:10.1111/j.1365-2990.2006.00733.x
Acknowledgments
This work was supported by grant support from Science Foundation Ireland (Grant No. 10/RFP/NES2786) (GO’K).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Collins, L.M., Adriaanse, L.J., Theratile, S.D. et al. Class-IIa Histone Deacetylase Inhibition Promotes the Growth of Neural Processes and Protects Them Against Neurotoxic Insult. Mol Neurobiol 51, 1432–1442 (2015). https://doi.org/10.1007/s12035-014-8820-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8820-8