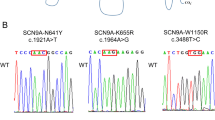
Abstract
Mutations in the sodium channel gene, SCN1A (NaV1.1), have been linked to a spectrum of epilepsy syndromes, and many of these mutations occur in the pore region of the channel. Electrophysiological characterization has revealed that most SCN1A mutations in the pore region result in complete loss of function. SCN3A mutations have also been identified in patients with epilepsy; however, mutations in this pore region maintain some degree of electrophysiological function. It is thus speculated that compared to SCN3A disruptions, SCN1A mutations have a more pronounced effect on electrophysiological function. In this study, we identified a novel mutation, N302S, in the SCN3A pore region of a child with epilepsy. To investigate if mutations at the pore regions of SCN3A and SCN1A have different impacts on channel function, we studied the electrophysiological properties of N302S in NaV1.3 and its homologous mutation (with the same amino acid substitution) in NaV1.1 (N301S). Functional analysis demonstrated that SCN1A-N301S had no measurable sodium current, indicating a complete loss of function, while SCN3A-N302S slightly reduced channel activity. This observation indicates that the same pore region mutation affects SCN1A more than SCN3A. Our study further revealed a huge difference in electrophysiological function between SCN1A and SCN3A mutations in the pore region; this might explain the more common SCN1A mutations detected in patients with epilepsy and the more severe phenotypes associated with these mutations.



Similar content being viewed by others
References
Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26:13–25
Saleh S, Yeung SY, Prestwich S, Pucovsky V, Greenwood I (2005) Electrophysiological and molecular identification of voltage-gated sodium channels in murine vascular myocytes. J Physiol 568:155–169
Catterall WA (1988) Molecular properties of voltage-sensitive sodium and calcium channels. Braz J Med Biol Res 21:1129–1144
Isom LL, De Jongh KS, Catterall WA (1994) Auxiliary subunits of voltage-gated ion channels. Neuron 12:1183–1194
Brackenbury WJ, Djamgoz MB, Isom LL (2008) An emerging role for voltage-gated Na + channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist 14:571–583
Whitaker WR, Clare JJ, Powell AJ, Chen YH, Faull RL, Emson PC (2000) Distribution of voltage-gated sodium channel alpha-subunit and beta-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. J Comp Neurol 422:123–139
Whitaker WR, Faull RL, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ (2001) Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res Mol Brain Res 88:37–53
Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA (2006) Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 9:1142–1149
Beckh S, Noda M, Lübbert H, Numa S (1989) Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J 8:3611–3616
Liao WP, Shi YW, Long YS, Zeng Y, Li T, Yu MJ, Su T, Deng P, Lei ZG, Xu SJ, Deng WY, Liu XR, Sun WW, Yi YH, Xu ZC, Duan S (2010) Partial epilepsy with antecedent febrile seizures and seizure aggravation by antiepileptic drugs: associated with loss of function of NaV1.1. Epilepsia 51:1669–1678
Lossin C, Rhodes TH, Desai RR, Vanoye CG, Wang D, Carniciu S, Devinsky O, George AL Jr (2003) Epilepsy-associated dysfunction in the voltage-gated neuronal sodium channel SCN1A. J Neurosci 23:11289–11295
Ohmori I, Kahlig KM, Rhodes TH, Wang DW, George AL Jr (2006) Nonfunctional SCN1A is common in severe myoclonic epilepsy of infancy. Epilepsia 47:1636–1642
Rhodes TH, Lossin C, Vanoye CG, Wang DW, George AL Jr (2004) Proc Natl Acad Sci U S A 101:11147–11152
Rhodes TH, Vanoye CG, Ohmori I, Ogiwara I, Yamakawa K, George AL Jr (2005) Sodium channel dysfunction in intractable childhood epilepsy with generalized tonic-clonic seizures. J Physiol 569:433–445
Sugawara T, Tsurubuchi Y, Fujiwara T, Mazaki-Miyazaki E, Nagata K, Montal M, Inoue Y, Yamakawa K (2003) NaV1.1 channels with mutations of severe myoclonic epilepsy in infancy display attenuated currents. Epilepsy Res 54:201–207
Sugiura Y, Ogiwara I, Hoshi A, Yamakawa K, Ugawa Y (2012) Different degrees of loss of function between GEFS + and SMEI NaV1.1 missense mutants at the same residue induced by rescuable folding defects. Epilepsia 53:111–114
Volkers L, Kahlig KM, Verbeek NE, Das JH, van Kempen MJ, Stroink H, Augustijn P, van Nieuwenhuizen O, Lindhout D, George AL Jr, Koeleman BP, Rook MB (2011) NaV 1.1 dysfunction in genetic epilepsy with febrile seizures-plus or Dravet syndrome. Eur J Neurosci 34:1268–1275
Holland KD, Kearney JA, Glauser TA, Buck G, Keddache M, Blankston JR, Glaaser IW, Kass RS, Meisler MH (2008) Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci Lett 433:65–70
Vanoye CG, Gurnett CA, Holland KD, George AL Jr, Kearney JA (2014) Novel SCN3A variants associated with focal epilepsy in children. Neurobiol Dis 62:313–322
Lossin C, Wang DW, Rhodes TH, Vanoye CG, George AL Jr (2002) Molecular basis of an inherited epilepsy. Neuron 34:877–884
Estacion M, Gasser A, Dib-Hajj SD, Waxman SG (2010) A sodium channel mutation linked to epilepsy increases ramp and persistent current of NaV1.3 and induces hyper excitability in hippocampal neurons. Exp Neurol 224:362–368
Lossin C (2009) A catalog of SCN1A variants. Brain Dev 31:114–130
Mulley JC, Scheffer IE, Petrou S, Dibbens LM, Berkovic SF, Harkin LA (2005) SCN1A mutations and epilepsy. Hum Mutat 25:535–542
Payandeh J, Scheuer T, Zheng N, Catterall WA (2011) The crystal structure of a voltage-gated sodium channel. Nature 475:353–358
Zhang X, Yan N (2013) The conformational shifts of the voltage sensing domains between NaVRh and NaVAb. Cell Res 23:444–447
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant no. 81271434 to W.P.L. and 81071045 to Y.W.S.), the Yangcheng Scholar Research Project of Guangzhou Municipal College (Grant no. 12A016S to W.P.L.), the Guangdong Natural Science Foundation (Grant no. S2012040007497 to Y.Z.), the Scientific Research of Guangzhou Municipal Colleges and Universities (Grant no. 12C168 to Y.Z.) and the Scientific Research of Guangzhou Medical Universities (Grant no. 11C47 to Y.Z.) supported this study. We are grateful to the He Shanheng Charity Foundation for contributing to the development of this institute.
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, YJ., Shi, YW., Xu, HQ. et al. Electrophysiological Differences between the Same Pore Region Mutation in SCN1A and SCN3A . Mol Neurobiol 51, 1263–1270 (2015). https://doi.org/10.1007/s12035-014-8802-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8802-x