Abstract
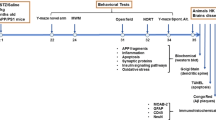
Alzheimer’s disease (AD) can be divided into sporadic AD (SAD) and familial AD (FAD). Most AD cases are sporadic and result from multiple etiologic factors, including environmental, genetic, and metabolic factors, whereas FAD is caused by mutations in the presenilins or amyloid-β (Aβ) precursor protein (APP) genes. A commonly used animal model for AD is the 3xTg-AD transgenic mouse model, which harbors mutated presenilin 1, APP, and tau genes and thus represents a model of FAD. There is an unmet need in the field to characterize animal models representing different AD mechanisms, so that potential drugs for SAD can be evaluated preclinically in these animal models. A mouse model generated by intracerebroventricular (icv) administration of streptozocin (STZ), the icv-STZ mouse, shows many aspects of SAD. In this study, we compared the non-cognitive and cognitive behaviors as well as biochemical and immunohistochemical alterations between the icv-STZ mouse and the 3xTg-AD mouse. We found that both mouse models showed increased exploratory activity as well as impaired learning and spatial memory. Both models also demonstrated neuroinflammation, altered synaptic proteins and insulin/IGF-1 (insulin-like growth factor-1) signaling, and increased hyperphosphorylated tau in the brain. The most prominent brain abnormality in the icv-STZ mouse was neuroinflammation, and in the 3xTg-AD mouse it was elevation of hyperphosphorylated tau. These observations demonstrate the behavioral and neuropathological similarities and differences between the icv-STZ mouse and the 3xTg-AD mouse models and will help guide future studies using these two mouse models for the development of AD drugs.






Similar content being viewed by others
References
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 83(13):4913–4917
Iqbal K, Grundke-Iqbal I (2010) Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimers Dement 6(5):420–424. doi:10.1016/j.jalz.2010.04.006
Waring SC, Rosenberg RN (2008) Genome-wide association studies in Alzheimer disease. Arch Neurol 65(3):329–334. doi:10.1001/archneur.65.3.329
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39(3):409–421
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM (2003) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24(8):1063–1070
Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ (2005) Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer’s disease mice. J Neuroinflammation 2:23. doi:10.1186/1742-2094-2-23
Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM (2005) Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 45(5):675–688. doi:10.1016/j.neuron.2005.01.040
Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, McGaugh JL, LaFerla FM (2007) Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis 28(1):76–82. doi:10.1016/j.nbd.2007.06.013
Mastrangelo MA, Bowers WJ (2008) Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci 9:81. doi:10.1186/1471-2202-9-81
Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S (2007) Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem 101(3):757–770. doi:10.1111/j.1471-4159.2006.04368.x
Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P (2006) Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem 96(4):1005–1015. doi:10.1111/j.1471-4159.2005.03637.x
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50(6):537–546
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A (2003) Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 30(8):1104–1113. doi:10.1007/s00259-003-1194-1
Heiss WD, Szelies B, Kessler J, Herholz K (1991) Abnormalities of energy metabolism in Alzheimer’s disease studied with PET. Ann N Y Acad Sci 640:65–71
Duarte AI, Moreira PI, Oliveira CR (2012) Insulin in central nervous system: more than just a peripheral hormone. J Aging Res 2012:384017. doi:10.1155/2012/384017
Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2011) Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 225(1):54–62. doi:10.1002/path.2912
Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis 7(1):63–80
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122(4):1316–1338. doi:10.1172/jci59903
Salkovic-Petrisic M, Hoyer S (2007) Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: an experimental approach. J Neural Transm Suppl 72:217–233
Salkovic-Petrisic M, Osmanovic J, Grunblatt E, Riederer P, Hoyer S (2009) Modeling sporadic Alzheimer’s disease: the insulin resistant brain state generates multiple long-term morphobiological abnormalities including hyperphosphorylated tau protein and amyloid-beta. J Alzheimers Dis 18(4):729–750. doi:10.3233/jad-2009-1184
Tatebayashi Y, Iqbal K, Grundke-Iqbal I (1999) Dynamic regulation of expression and phosphorylation of tau by fibroblast growth factor-2 in neural progenitor cells from adult rat hippocampus. J Neurosci 19(13):5245–5254
Pei JJ, Gong CX, Iqbal K, Grundke-Iqbal I, Wu QL, Winblad B, Cowburn RF (1998) Subcellular distribution of protein phosphatases and abnormally phosphorylated tau in the temporal cortex from Alzheimer’s disease and control brains. J Neural Transm 105(1):69–83
Sargolini F, Roullet P, Oliverio A, Mele A (2003) Effects of intra-accumbens focal administrations of glutamate antagonists on object recognition memory in mice. Behav Brain Res 138(2):153–163
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297(5868):681–683
Bensadoun A, Weinstein D (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70(1):241–250
Blanchard J, Wanka L, Tung YC, Cardenas-Aguayo Mdel C, LaFerla FM, Iqbal K, Grundke-Iqbal I (2010) Pharmacologic reversal of neurogenic and neuroplastic abnormalities and cognitive impairments without affecting Abeta and tau pathologies in 3xTg-AD mice. Acta Neuropathol 120(5):605–621
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167
Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2(1):a006346. doi:10.1101/cshperspect.a006346
Correia SC, Santos RX, Perry G, Zhu X, Moreira PI, Smith MA (2011) Insulin-resistant brain state: the culprit in sporadic Alzheimer’s disease? Ageing Res Rev 10(2):264–273. doi:10.1016/j.arr.2011.01.001
Prickaerts J, Fahrig T, Blokland A (1999) Cognitive performance and biochemical markers in septum, hippocampus and striatum of rats after an i.c.v. injection of streptozotocin: a correlation analysis. Behav Brain Res 102(1–2):73–88
Gimenez-Llort L, Blazquez G, Canete T, Johansson B, Oddo S, Tobena A, LaFerla FM, Fernandez-Teruel A (2007) Modeling behavioral and neuronal symptoms of Alzheimer’s disease in mice: a role for intraneuronal amyloid. Neurosci Biobehav Rev 31(1):125–147. doi:10.1016/j.neubiorev.2006.07.007
Dou J, Cui C, Dufour F, Alkon DL, Zhao WQ (2003) Gene expression of alpha-endosulfine in the rat brain: correlative changes with aging, learning and stress. J Neurochem 87(5):1086–1100
Mayer G, Nitsch R, Hoyer S (1990) Effects of changes in peripheral and cerebral glucose metabolism on locomotor activity, learning and memory in adult male rats. Brain Res 532(1–2):95–100
Cerejeira J, Lagarto L, Mukaetova-Ladinska EB (2012) Behavioral and psychological symptoms of dementia. Front Neurol 3:73. doi:10.3389/fneur.2012.00073
Sterniczuk R, Antle MC, Laferla FM, Dyck RH (2010) Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 2. Behavioral and cognitive changes. Brain Res 1348:149–155. doi:10.1016/j.brainres.2010.06.011
Pinton S, da Rocha JT, Gai BM, Nogueira CW (2011) Sporadic dementia of Alzheimer’s type induced by streptozotocin promotes anxiogenic behavior in mice. Behav Brain Res 223(1):1–6. doi:10.1016/j.bbr.2011.04.014
McGeer EG, McGeer PL (2003) Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27(5):741–749. doi:10.1016/s0278-5846(03)00124-6
Simpson JE, Ince PG, Lace G, Forster G, Shaw PJ, Matthews F, Savva G, Brayne C, Wharton SB (2010) Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging 31(4):578–590. doi:10.1016/j.neurobiolaging.2008.05.015
Prickaerts J, De Vente J, Honig W, Steinbusch H, Ittersum MMV, Blokland A, Steinbusch HW (2000) Nitric oxide synthase does not mediate neurotoxicity after an i.c.v. injection of streptozotocin in the rat. J Neural Transm 107(7):745–766
Weinstock M, Shoham S (2004) Rat models of dementia based on reductions in regional glucose metabolism, cerebral blood flow and cytochrome oxidase activity. J Neural Transm 111(3):347–366. doi:10.1007/s00702-003-0058-y
Rodrigues L, Biasibetti R, Swarowsky A, Leite MC, Quincozes-Santos A, Quilfeldt JA, Achaval M, Goncalves CA (2009) Hippocampal alterations in rats submitted to streptozotocin-induced dementia model are prevented by aminoguanidine. J Alzheimers Dis 17(1):193–202. doi:10.3233/jad-2009-1034
Shoham S, Bejar C, Kovalev E, Schorer-Apelbaum D, Weinstock M (2007) Ladostigil prevents gliosis, oxidative–nitrative stress and memory deficits induced by intracerebroventricular injection of streptozotocin in rats. Neuropharmacology 52(3):836–843. doi:10.1016/j.neuropharm.2006.10.005
Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM (2006) Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis 9(1):13–33
Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM (2005) Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci 25(39):8843–8853. doi:10.1523/jneurosci.2868-05.2005
Takasu N, Komiya I, Asawa T, Nagasawa Y, Yamada T (1991) Streptozocin- and alloxan-induced H2O2 generation and DNA fragmentation in pancreatic islets. H2O2 as mediator for DNA fragmentation. Diabetes 40(9):1141–1145
Javed H, Khan MM, Ahmad A, Vaibhav K, Ahmad ME, Khan A, Ashafaq M, Islam F, Siddiqui MS, Safhi MM (2012) Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 210:340–352. doi:10.1016/j.neuroscience.2012.02.046
Isik AT, Celik T, Ulusoy G, Ongoru O, Elibol B, Doruk H, Bozoglu E, Kayir H, Mas MR, Akman S (2009) Curcumin ameliorates impaired insulin/IGF signalling and memory deficit in a streptozotocin-treated rat model. Age (Dordr) 31(1):39–49. doi:10.1007/s11357-008-9078-8
Saxena G, Patro IK, Nath C (2011) ICV STZ induced impairment in memory and neuronal mitochondrial function: a protective role of nicotinic receptor. Behav Brain Res 224(1):50–57. doi:10.1016/j.bbr.2011.04.039
Javed H, Khan MM, Khan A, Vaibhav K, Ahmad A, Khuwaja G, Ahmed ME, Raza SS, Ashafaq M, Tabassum R, Siddiqui MS, El-Agnaf OM, Safhi MM, Islam F (2011) S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res 1389:133–142. doi:10.1016/j.brainres.2011.02.072
Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, Yousuf S, Ahmad A, Shrivastav P, Islam F (2009) Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res 1281:117–127. doi:10.1016/j.brainres.2009.04.010
Ishrat T, Hoda MN, Khan MB, Yousuf S, Ahmad M, Khan MM, Ahmad A, Islam F (2009) Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT). Eur Neuropsychopharmacol 19(9):636–647. doi:10.1016/j.euroneuro.2009.02.002
Dhull DK, Jindal A, Dhull RK, Aggarwal S, Bhateja D, Padi SS (2012) Neuroprotective effect of cyclooxygenase inhibitors in ICV-STZ induced sporadic Alzheimer’s disease in rats. J Mol Neurosci 46(1):223–235. doi:10.1007/s12031-011-9583-6
Arendt T (2009) Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol 118(1):167–179. doi:10.1007/s00401-009-0536-x
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30(4):572–580. doi:10.1002/ana.410300410
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298(5594):789–791. doi:10.1126/science.1074069
Barnes CA (1999) Do synaptic markers provide a window on synaptic effectiveness in the aged hippocampus? Neurobiol Aging 20(3):349–351, discussion 359–360
Nicolle MM, Gallagher M, McKinney M (1999) No loss of synaptic proteins in the hippocampus of aged, behaviorally impaired rats. Neurobiol Aging 20(3):343–348
Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX (2009) Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: implication for Alzheimer’s disease. Am J Pathol 175(5):2089–2098. doi:10.2353/ajpath.2009.090157
Oddo S, Caccamo A, Cheng D, Jouleh B, Torp R, LaFerla FM (2007) Genetically augmenting tau levels does not modulate the onset or progression of Abeta pathology in transgenic mice. J Neurochem 102(4):1053–1063. doi:10.1111/j.1471-4159.2007.04607.x
Salkovic-Petrisic M, Osmanovic-Barilar J, Bruckner MK, Hoyer S, Arendt T, Riederer P (2011) Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: a long-term follow up study. J Neural Transm 118(5):765–772. doi:10.1007/s00702-011-0651-4
Acknowledgments
We thank Ms. J. Murphy for secretarial assistance. This work was supported in part by the New York State Office for People with Developmental Disabilities as well as grants from the National Institutes of Health (R01 AG027429, R03 TW008123), the U.S. Alzheimer’s Association (IIRG-10-170405 and IIRG-10-173154), the National Natural Science Foundation of China (30901386), and the Wuhan Science and Technology Bureau, China (200960323132). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yanxing Chen and Zhihou Liang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, Y., Liang, Z., Blanchard, J. et al. A Non-transgenic Mouse Model (icv-STZ Mouse) of Alzheimer’s Disease: Similarities to and Differences from the Transgenic Model (3xTg-AD Mouse). Mol Neurobiol 47, 711–725 (2013). https://doi.org/10.1007/s12035-012-8375-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8375-5