Abstract
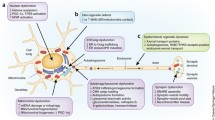
The discovery of α-synuclein has had profound implications concerning our understanding of Parkinson’s disease (PD) and other neurodegenerative disorders characterized by α-synuclein accumulation. In fact, as compared with pre-α-synuclein times, a “new” PD can now be described as a whole-body disease in which a progressive spreading of α-synuclein pathology underlies a wide spectrum of motor as well as nonmotor clinical manifestations. Not only is α-synuclein accumulation a pathological hallmark of human α-synucleinopathies but increased protein levels are sufficient to trigger neurodegenerative processes. α-Synuclein elevations could also be a mechanism by which disease risk factors (e.g., aging) increase neuronal vulnerability to degeneration. An important corollary to the role of enhanced α-synuclein in PD pathogenesis is the possibility of developing α-synuclein-based biomarkers and new therapeutics aimed at suppressing α-synuclein expression. The use of in vitro and in vivo experimental models, including transgenic mice overexpressing α-synuclein and animals with viral vector-mediated α-synuclein transduction, has helped clarify pathogenetic mechanisms and therapeutic strategies involving α-synuclein. These models are not devoid of significant limitations, however. Therefore, further pursuit of new clues on the cause and treatment of PD in this post-α-synuclein era would benefit substantially from the development of improved research paradigms of α-synuclein elevation.



Similar content being viewed by others
References
Holdorff B (2002) Friedrich Heinrich Lewy (1885–1950) and his work. J Hist Neurosci 11:19–28
Hornykiewicz O (2010) A brief history of levodopa. J Neurol 257:S249–S252
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H et al (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A 95:6469–6473
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Dickson DW, Uchikado H, Fujishiro H, Tsuboi Y (2010) Evidence in favor of Braak staging of Parkinson’s disease. Mov Disord 25(Suppl 1):S78–S82
Braak H, de Vos RA, Bohl J, Del Tredici K (2006) Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72
Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H (2010) Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol 119:703–713
Galvin JE, Lee VM, Trojanowski JQ (2001) Synucleinopathies: clinical and pathological implications. Arch Neurol 58:186–190
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM et al (2000) Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252
Kurz A, Double KL, Lastres-Becker I, Tozzi A, Tantucci M, Bockhart V, Bonin M, Garcia-Arencibia M et al (2010) A53T-α-synuclein overexpression impairs dopamine signaling and striatal synaptic plasticity in old mice. PLoS One 5:e11464
Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC (2010) α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329:1663–1667
Uversky VN (2007) Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J Neurochem 103:17–37
Rochet JC, Conway KA, Lansbury PT Jr (2000) Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse α-synuclein. Biochemistry 39:10619–10626
Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT Jr (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418:291
Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr (2001) Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science 294:1346–1349
Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J et al (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein α-synuclein. J Biol Chem 286:20710–20726
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305:1292–1295
Hettiarachchi NT, Parker A, Dallas ML, Pennington K, Hung CC, Pearson HA, Boyle JP, Robinson P et al (2009) α-Synuclein modulation of Ca2+ signaling in human neuroblastoma (SH-SY5Y) cells. J Neurochem 111:1192–1201
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT et al (1998) Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet 18:106–108
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J et al (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–173
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T et al (2003) α-Synuclein locus triplication causes Parkinson’s disease. Science 302:841
Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K et al (2004) Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann Neurol 55:174–179
Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A et al (2000) Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287:1265–1269
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L et al (2004) α-Synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169
Ross OA, Braithwaite AT, Skipper LM, Kachergus J, Hulihan MM, Middleton FA, Nishioka K, Fuchs J et al (2008) Genomic investigation of α-synuclein multiplication and parkinsonism. Ann Neurol 63:743–750
Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P et al (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41:1308–1312
Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T et al (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet 41:1303–1307
Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK et al (2006) Collaborative analysis of α-synuclein gene promoter variability and Parkinson disease. JAMA 296:661–670
Fuchs J, Tichopad A, Golub Y, Munz M, Schweitzer KJ, Wolf B, Berg D, Mueller JC et al (2008) Genetic variability in the SNCA gene influences α-synuclein levels in the blood and brain. FASEB J 22:1327–1334
Di Monte DA (2003) The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol 2:531–538
Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM (2003) Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 157:1015–1022
Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS et al (2011) Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 119:866–872
Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA (2003) Head trauma preceding PD: a case–control study. Neurology 60:1610–1615
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980
Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S (2000) α-Synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem 74:721–729
Purisai MG, McCormack AL, Langston WJ, Johnston LC, Di Monte DA (2005) α-Synuclein expression in the substantia nigra of MPTP-lesioned non-human primates. Neurobiol Dis 20:898–906
McCormack AL, Mak SK, Shenasa M, Langston WJ, Forno LS, Di Monte DA (2008) Pathologic modifications of α-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J Neuropathol Exp Neurol 67:793–802
Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA (2002) The herbicide paraquat causes up-regulation and aggregation of α-synuclein in mice: paraquat and α-synuclein. J Biol Chem 277:1641–1644
Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA (2010) Lysosomal degradation of α-synuclein in vivo. J Biol Chem 285:13621–13629
Gatto NM, Rhodes SL, Manthripragada AD, Bronstein J, Cockburn M, Farrer M, Ritz B (2010) α-Synuclein gene may interact with environmental factors in increasing risk of Parkinson’s disease. Neuroepidemiology 35:191–195
Goldman SM, Kamel F, Ross GW, Jewell SA, Bhudhikanok GS, Umbach D, Marras C, Hauser RA et al (2012) Head injury, α-synuclein Rep1 and Parkinson’s disease. Ann Neurol 71:40–48
Uryu K, Giasson BI, Longhi L, Martinez D, Murray I, Conte V, Nakamura M, Saatman K et al (2003) Age-dependent synuclein pathology following traumatic brain injury in mice. Exp Neurol 184:214–224
Martinez-Vicente M, Cuervo AM (2007) Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol 6:352–361
Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334:345–348
German DC, Manaye KF, Sonsalla PK, Brooks BA (1992) Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci 648:42–62
Li W, Lesuisse C, Xu Y, Troncoso JC, Price DL, Lee MK (2004) Stabilization of α-synuclein protein with aging and familial Parkinson’s disease-linked A53T mutation. J Neurosci 24:7400–7409
Chu Y, Kordower JH (2007) Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis 25:134–149
McCormack A, Mak SK, Di Monte DA (2012) Increased α-synuclein phosphorylation and nitration in the aging promate substantia nigra. Cell Death Dis 3:e315
Mak SK, McCormack AL, Langston JW, Kordower JH, Di Monte DA (2009) Decreased α-synuclein expression in the aging mouse substantia nigra. Exp Neurol 220:359–365
Beyer K, Ariza A (2008) The therapeutical potential of α-synuclein antiaggregatory agents for dementia with Lewy bodies. Curr Med Chem 15:2748–2759
Chiba-Falek O, Kowalak JA, Smulson ME, Nussbaum RL (2005) Regulation of α-synuclein expression by poly (ADP ribose) polymerase-1 (PARP-1) binding to the NACP-Rep1 polymorphic site upstream of the SNCA gene. Am J Hum Genet 76:478–492
Hayashita-Kinoh H, Yamada M, Yokota T, Mizuno Y, Mochizuki H (2006) Down-regulation of α-synuclein expression can rescue dopaminergic cells from cell death in the substantia nigra of Parkinson’s disease rat model. Biochem Biophys Res Commun 341:1088–1095
Sapru MK, Yates JW, Hogan S, Jiang L, Halter J, Bohn MC (2006) Silencing of human α-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol 198:382–390
Lewis J, Melrose H, Bumcrot D, Hope A, Zehr C, Lincoln S, Braithwaite A, He Z et al (2008) In vivo silencing of α-synuclein using naked siRNA. Mol Neurodegener 3:19
McCormack AL, Mak SK, Henderson JM, Bumcrot D, Farrer MJ, Di Monte DA (2010) α-Synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PLoS One 5:e12122
Gorbatyuk OS, Li S, Nash K, Gorbatyuk M, Lewin AS, Sullivan LF, Mandel RJ, Chen W et al (2010) In vivo RNAi-mediated α-synuclein silencing induces nigrostriatal degeneration. Mol Ther 18:1450–1457
Martin JN, Wolken N, Brown T, Dauer WT, Ehrlich ME, Gonzalez-Alegre P (2011) Lethal toxicity caused by expression of shRNA in the mouse striatum: implications for therapeutic design. Gene Ther 18:666–673
Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA (2003) α-Synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci 23:3095–3099
Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC (2005) α-Synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123:383–396
Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP et al (2010) DJ-1 and α-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 133:713–726
Foulds PG, Mitchell JD, Parker A, Turner R, Green G, Diggle P, Hasegawa M, Taylor M et al (2011) Phosphorylated α-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. FASEB J 25:4127–4137
Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, Ishigami N, Tamaoka A et al (2010) Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75:1766–1772
Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH et al (2012) α-Synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord 27:709–715
Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N’Guyen JM, Chaumette T, Tasselli M et al (2010) Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One 5:e12728
Lee HJ, Patel S, Lee SJ (2005) Intravesicular localization and exocytosis of α-synuclein and its aggregates. J Neurosci 25:6016–6024
Feany MB, Bender WW (2000) A Drosophila model of Parkinson’s disease. Nature 404:394–398
Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T et al (2010) Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 29:3571–3589
Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A (2012) Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons. Neurobiol Dis 45:939–953
Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, Baker H, Ridley RM et al (2007) Long-term consequences of human α-synuclein overexpression in the primate ventral midbrain. Brain 130:799–815
Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E (2002) Differential neuropathological alterations in transgenic mice expressing α-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res 68:568–578
Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC et al (2006) Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human α-synuclein(1–120): implications for Lewy body disorders. J Neurosci 26:3942–3950
Wakamatsu M, Ishii A, Iwata S, Sakagami J, Ukai Y, Ono M, Kanbe D, Muramatsu S et al (2008) Selective loss of nigral dopamine neurons induced by overexpression of truncated human α-synuclein in mice. Neurobiol Aging 29:574–585
Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E (2001) β-Synuclein inhibits α-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron 32:213–223
Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D et al (2001) Lack of nigral pathology in transgenic mice expressing human α-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis 8:535–539
Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK (2004) Risk factors for dopaminergic neuron loss in human α-synuclein transgenic mice. Eur J Neurosci 19:845–854
Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okoch M, Leimer U et al (2000) Subcellular localization of wild-type and Parkinson’s disease-associated mutant α-synuclein in human and transgenic mouse brain. J Neurosci 20:6365–6373
van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K et al (2000) Neuropathology in mice expressing human α-synuclein. J Neurosci 20:6021–6029
Fleming SM, Salcedo J, Hutson CB, Rockenstein E, Masliah E, Levine MS, Chesselet MF (2006) Behavioral effects of dopaminergic agonists in transgenic mice overexpressing human wildtype α-synuclein. Neuroscience 142:1245–1253
Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF (2008) Olfactory deficits in mice overexpressing human wildtype α-synuclein. Eur J Neurosci 28:247–256
Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Horsten S, Schmidt T, Boy J, Kuhn M et al (2008) Neurodegeneration and motor dysfunction in a conditional model of Parkinson’s disease. J Neurosci 28:2471–2484
Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG et al (2002) Human α-synuclein-harboring familial Parkinson’s disease-linked Ala-53-Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A 99:8968–8973
Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T et al (1998) Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol 44:415–422
Piao YS, Wakabayashi K, Hayashi S, Yoshimoto M, Takahashi H (2000) Aggregation of α-synuclein/NACP in the neuronal and glial cells in diffuse Lewy body disease: a survey of six patients. Clin Neuropathol 19:163–169
Low K, Aebischer P (2012) Use of viral vectors to create animal models for Parkinson’s disease. Neurobiol Dis 48:189–201
Kim SR, Ries V, Cheng HC, Kareva T, Oo TF, Yu WH, Duff K, Kholodilov N et al (2011) Age and α-synuclein expression interact to reveal a dependence of dopaminergic axons on endogenous Akt/PKB signaling. Neurobiol Dis 44:215–222
Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, Mandel RJ, Muzyczka N (2008) The phosphorylation state of Ser-129 in human α-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci U S A 105:763–768
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A (2002) Parkinson-like neurodegeneration induced by targeted overexpression of α-synuclein in the nigrostriatal system. J Neurosci 22:2780–2791
Decressac M, Ulusoy A, Mattsson B, Georgievska B, Romero-Ramos M, Kirik D, Bjorklund A (2011) GDNF fails to exert neuroprotection in a rat α-synuclein model of Parkinson’s disease. Brain 134:2302–2311
Van der Perren A, Toelen J, Carlon M, Van den Haute C, Coun F, Heeman B, Reumers V, Vandenberghe LH et al (2011) Efficient and stable transduction of dopaminergic neurons in rat substantia nigra by rAAV 2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8 and 2/9. Gene Ther 18:517–527
Korecka J, Schouten M, Eggers R, Ulusoy A, Bossers K, Verhaagen J (2011) Viral gene therapy. In: Xu K (ed) Comparison of AAV serotypes for gene delivery to dopaminergic neurons in the substantia nigra. InTech, Rijeka, pp 205–224
Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P (2002) α-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci USA 99:10813–10818
Ulusoy A, Febbraro F, Jensen PH, Kirik D, Romero-Ramos M (2010) Co-expression of C-terminal truncated α-synuclein enhances full-length α-synuclein-induced pathology. Eur J Neurosci 32:409–422
Galvin JE, Uryu K, Lee VM, Trojanowski JQ (1999) Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains α-, β-, and γ-synuclein. Proc Natl Acad Sci U S A 96:13450–13455
Chung CY, Koprich JB, Siddiqi H, Isacson O (2009) Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV α-synucleinopathy. J Neurosci 29:3365–3373
Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M et al (2011) Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun 2:440
Acknowledgments
The authors thank Dr. Sarah Jewell for her comments on the manuscript. This work was supported by the Centres of Excellence in Neurodegeneration Research (CoEN) and the Blanche A. Paul Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulusoy, A., Di Monte, D.A. α-Synuclein Elevation in Human Neurodegenerative Diseases: Experimental, Pathogenetic, and Therapeutic Implications. Mol Neurobiol 47, 484–494 (2013). https://doi.org/10.1007/s12035-012-8329-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8329-y