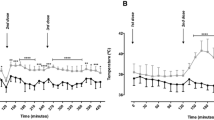
Abstract
“Ecstasy” [(±)-3,4-methylenedioxymethamphetamine, MDMA, XTC, X, E] is a psychoactive recreational hallucinogenic substance and a major worldwide drug of abuse. Several reports raised the concern that MDMA has the ability to induce neurotoxic effects both in laboratory animals and humans. Despite more than two decades of research, the mechanisms by which MDMA is neurotoxic are still to be fully elucidated. MDMA induces serotonergic terminal loss in rats and also in some mice strains, but also a broader neuronal degeneration throughout several brain areas such as the cortex, hippocampus, and striatum. Meanwhile, in human “ecstasy” abusers, there are evidences for deficits in seronergic biochemical markers, which correlate with long-term impairments in memory and learning. There are several factors that contribute to MDMA-induced neurotoxicity, namely, hyperthermia, monoamine oxidase metabolism of dopamine and serotonin, dopamine oxidation, the serotonin transporter action, nitric oxide, and the formation of peroxinitrite, glutamate excitotoxicity, serotonin 2A receptor agonism, and, importantly, the formation of MDMA neurotoxic metabolites. The present review covered the following topics: history and epidemiology, pharmacological mechanisms, metabolic pathways and the influence of isoenzyme genetic polymorphisms, as well as the acute effects of MDMA in laboratory animals and humans, with a special focus on MDMA-induced neurotoxic effects at the cellular and molecular level. The main aim of this review was to contribute to the understanding of the cellular and molecular mechanisms involved in MDMA neurotoxicity, which can help in the development of therapeutic approaches to prevent or treat the long-term neuropsychiatric complications of MDMA abuse in humans.










Similar content being viewed by others
Abbreviations
- Amph:
-
Amphetamine
- AMPT:
-
α-Methyl-p-tyrosine
- ATP:
-
Adenosine triphosphate
- AUC:
-
Area under the curve
- C max :
-
Maximum concentration
- CNS:
-
Central nervous system
- COMT:
-
Catechol-O-methyltransferase
- CSF:
-
Cerebrospinal fluid
- CTX:
-
Cortex
- CYP:
-
Cytochrome P450
- DA:
-
Dopamine
- DAT:
-
Dopamine transporter
- DHT:
-
Dihydroxytriptamine
- DOI:
-
(±)-2,5-Dimethoxy-4-iodoamphetamine
- EC50 :
-
Effective concentration 50%
- EU:
-
European Union
- GABA:
-
Gamma-aminobutyric acid
- GFAP:
-
Glial fibrillary acidic protein
- GLU:
-
Glutamate
- GSH:
-
Glutathione
- GST:
-
Glutathione S-transferase
- γ-GT:
-
gamma-glutamyl transpeptidase or gamma-glutamyltransferase
- 5-HIAA:
-
5-Hydroxyindoleacetic acid
- HIP:
-
Hippocampus
- HMA:
-
4-Hydroxy-3-methoxyamphetamine, 3-O-Me-α-MeDA
- HMMA:
-
4-Hydroxy-3-methoxymethamphetamine, 3-O-Me-N-Me-α-MeDA
- HO● :
-
Hydroxyl radical
- H2O2 :
-
Hydrogen peroxide
- 5-HT:
-
5-Hydroxytriptamine, serotonin
- 5-HTT:
-
Serotonin transporter
- HVA:
-
4-Hydroxy-3-methoxyphenylacetic acid, homovanillic acid
- i.p.:
-
Intraperitoneal
- i.v.:
-
Intravenous
- iCa2+ :
-
Intracellular calcium
- ICV:
-
Intracerebroventricular
- K e :
-
Elimination constant
- KO:
-
Knockout
- MAO:
-
Monoamine oxidase
- MDA:
-
(±)-3,4-Methylenedioxyamphetamine
- MDMA:
-
(±)-3,4-Methylenedioxymethamphetamine, “ecstasy”
- α-MeDA:
-
α-Methyldopamine, 3,4-Dihydroxyamphetamine, HHA
- N-Me-α-MeDA:
-
N-methyl-α-methyldopamine, 3,4-Dihydroxymethamphetamine, HHMA
- Meth:
-
Methamphetamine
- MK-801:
-
Dizocilpine
- NAC:
-
N-acetylcysteine
- NE:
-
Norepinephrine
- NET:
-
Norepinephrine transporter
- NMDA:
-
N-methyl-d-aspartic acid
- l-NAME:
-
\(N_\omega \)-nitro-l-arginine methyl ester
- l-NNA:
-
\(N_\omega \)-nitro-l-arginine
- NO:
-
Nitric oxide
- NO● :
-
Nitric oxide radical
- O2 ●− :
-
Superoxide anion
- ONOO− :
-
Peroxynitrite
- p.o.:
-
Per os
- PBN:
-
α-Phenyl-N-tert-butyl nitrone
- PND:
-
Postnatal day
- PET:
-
Positron emission tomography
- PKC:
-
Protein kinase C
- R-96544:
-
(2R,4R)-5-[2-[2-[2-(3-Methoxyphenyl)ethyl]phenoxy]ethyl]-1-methyl-3-pyrrolidinol hydrochloride
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- s.c.:
-
Subcutaneous
- –SH:
-
Sulfhydryl
- SPECT:
-
Single photon emission computed tomography
- SULT:
-
Sulfotransferase
- t 1/2 :
-
Elimination half-life
- T max :
-
Median time to maximum concentration
- T-4,5-D:
-
Tryptamine-4,5-dione
- TPH:
-
Tryptophan hydroxylase
- UGT:
-
UDP-glucuronosyltransferase
- VMAT:
-
Vesicular monoamine transporter
- WT:
-
Wild type
References
Freudenmann RW, Öxler F, Bernschneider-Reif S (2006) The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents. Addiction 101:1241–1245
Pentney AR (2001) An exploration of the history and controversies surrounding MDMA and MDA. J Psychoactive Drugs 33:213–221
Shulgin AT, Nichols DE (1978) Characterization of three new psychotomimetics. Pergamon, New York
Greer G, Strassman RJ (1985) Information on “ecstasy”. Am J Psychiatry 142:1391
Grinspoon L, Bakalar JB (1986) Can drugs be used to enhance the psychotherapeutic process. Am J Psychotherapy 40:393–404
Great Britain (2008) The misuse of drugs Act 1971 (amendment) order 2008. The Stationery Office
Saunders N (1993) E for ecstasy. Saunders, London, UK
DEA (2008) Orange book. US Department of Justice, Drug Enforcement Administration (DEA)
Check E (2004) Psychedelic drugs: the ups and downs of ecstasy. Nature 429:126–128
Doblin R (2002) A clinical plan for MDMA (ecstasy) in the treatment of posttraumatic stress disorder (PTSD): partnering with the FDA. J Psychoactive Drugs 34:185–194
Sessa B (2005) Can psychedelics have a role in psychiatry once again. Br J Psychiatry 186:457–458
Parrott AC (2007) The psychotherapeutic potential of MDMA (3,4-methylenedioxymethamphetamine): an evidence-based review. Psychopharmacology 191:181–193
Morton J (2005) Ecstasy: pharmacology and neurotoxicity. Curr Opin Pharmacol 5:79–86
EMCDDA (2007) Annual report 2007: the state of the drugs problem in Europe. European Monitoring Centre for Drugs and Drug Addiction, Lisbon
UNODC (2007) World drug report. United Nations Office on Drugs and Crime, Vienna
Cole JC, Sumnall HR (2003) Altered states: the clinical effects of ecstasy. Pharmacol Ther 98:35–58
Parrott AC (2004) Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology 173:234–241
Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE (2008) Monitoring the future national survey results on drug use, 1975–2007, vol I: secondary school students.. National Institute on Drug Abuse, Bethesda, MD
Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE (2008) Monitoring the future national survey results on drug use, 1975–2007, vol ii: college students and adults ages 19–45. National Institute on Drug Abuse, Bethesda, MD
Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE (2008) Monitoring the future national results on adolescent drug use: overview of key findings, 2007. National Institute on Drug Abuse, Bethesda, MD
Schmidt CJ, Levin JA, Lovenberg W (1987) In vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem Pharmacol 36:747–755
Johnson MP, Hoffman AJ, Nichols DE (1986) Effects of the enantiomers of MDA, MDMA and related analogues on [3H] serotonin and [3H]dopamine release from superfused rat brain slices. Eur J Pharmacol 132:269–276
Berger UV, Gu XF, Azmitia EC (1992) The substituted amphetamines 3,4-methylenedioxymethamphetamine, methamphetamine, p-chloroamphetamine and fenfluramine induce 5-hydroxytryptamine release via a common mechanism blocked by fluoxetine and cocaine. Eur J Pharmacol 215:153–160
Crespi D, Mennini T, Gobbi M (1997) Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol 121:1735–1743
Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39:32–41
Montgomery T, Buon C, Eibauer S, Guiry PJ, Keenan AK, McBean GJ (2007) Comparative potencies of 3,4-methylenedioxymethamphetamine (MDMA) analogues as inhibitors of [3H]noradrenaline and [3H]5-HT transport in mammalian cell lines. Br J Pharmacol 152:1121–1130
Han D, Gu H (2006) Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 6:6
Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB (2005) N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or “ecstasy”). Neuropsychopharmacology 30:550–560
Yamamoto BK, Spanos LJ (1988) The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. Eur J Pharmacol 148:195–203
Gudelsky GA, Nash JF (1996) Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin–dopamine interactions. J Neurochem 66:243–249
Ramamoorthy S, Blakely RD (1999) Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science 285:763–766
Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A (2005) Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci 102:3495–3500
Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci 15:4102–4108
Azmitia EC, Murphy RB, Whitaker-Azmitia PM (1990) MDMA (ecstasy) effects on cultured serotonergic neurons: evidence for CA2(+)-dependent toxicity linked to release. Brain Res 26:97–103
Wichems CH, Hollingsworth CK, Bennett BA (1995) Release of serotonin induced by 3,4-methylenedioxymethamphetamine (MDMA) and other substituted amphetamines in cultured fetal raphe neurons: further evidence for calcium-independent mechanisms of release. Brain Res 695:10–18
Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB (2006) Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther 319:237–246
Fleckenstein AE, Hanson GR (2003) Impact of psychostimulants on vesicular monoamine transporter function. Eur J Pharmacol 479:283–289
Leonardi ET, Azmitia EC (1994) MDMA (ecstasy) inhibition of MAO type A and type B: comparisons with fenfluramine and fluoxetine (Prozac). Neuropsychopharmacology 10:231–238
Cervinski MA, Foster JD, Vaughan RA (2005) Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem 280:40442–40449
Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S (2005) Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol 67:2077–2087
O'Shea E, Esteban B, Camarero J, Green AR, Colado MI (2001) Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (“ecstasy”) on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology 40:65–74
Nichols DE (2004) Hallucinogens. Pharmacol Ther 101:131–181
Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, Titeler M (1989) Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology 98:495–499
Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB (1988) Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol 149:159–163
Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA (1994) Effect of the r(2) and s(1) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2a or 5-HT2c receptors. Neurosci Lett 177:111–115
Baker LE, Broadbent J, Michael EK, Matthews PK, Metosh CA, Saunders RB, West WB, Appel JB (1995) Assessment of the discriminative stimulus effects of the optical isomers of ecstasy (3,4-methylenedioxymethamphetamine; MDMA). Behav Pharmacol 6:263–275
Callahan PM, Appel JB (1988) Differences in the stimulus properties of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine in animals trained to discriminate hallucinogens from saline. J Pharmacol Exp Ther 246:866–870
Broadbent J, Appel JB, Michael EK, Ricker JH (1992) Discriminative stimulus effects of the optical isomers of 3,4-methylenedioxyamphetamine (MDA). Behav Pharmacol 3:443–454
Baker LE, Taylor MM (1997) Assessment of the MDA and MDMA optical isomers in a stimulant–hallucinogen discrimination. Pharmacol Biochem Behav 57:737–748
Chu T, Kumagai Y, DiStefano EW, Cho AK (1996) Disposition of methylenodioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol 51:789–796
Johnson EA, O’Callaghan JP, Miller DB (2004) Brain concentrations of d-MDMA are increased after stress. Psychopharmacology 173:278–286
Lavelle A, Honner V, Docherty JR (1999) Investigation of the prejunctional [alpha]2-adrenoceptor mediated actions of MDMA in rat atrium and vas deferens. Br J Pharmacol 128:975–980
Fischer HS, Zernig G, Schatz DS, Humpel C, Saria A (2000) MDMA (‘ecstasy’) enhances basal acetylcholine release in brain slices of the rat striatum. Eur J Neurosci 12:1385–1390
Garcia-Ratés S, Camarasa J, Escubedo E, Pubill D (2007) Methamphetamine and 3,4-methylenedioxymethamphetamine interact with central nicotinic receptors and induce their up-regulation. Toxicol Appl Pharmacol 223:195–205
de la Torre R, Farré M, Navarro M, Pacifici R, Zuccaro P, Pichini S (2004) Clinical pharmacokinetics of amfetamine and related substances monitoring in conventional and non-conventional matrices. Clin Pharmacokinet 43:157–185
de la Torre R, Farre M, Ortuno J, Mas M, Brenneisen R, Roset PN, Segura J, Cami J (2000) Non-linear pharmacokinetics of MDMA (“ecstasy”) in humans. Br J Clin Pharmacol 49:104–109
Delaforge M, Jaouen M, Bouille G (1999) Inhibitory metabolite complex formation of methylenedioxymethamphetamine with rat and human cytochrome p450: particular involvement of cyp2d. Environ Pharmacol Toxicol 7:153–158
Wu D, Otton SV, Inaba T, Kalow W, Sellers EM (1997) Interactions of amphetamine analogs with human liver cyp2d6. Biochem Pharmacol 53:1605–1612
Heydari A, Yeo KR, Lennard MS, Ellis SW, Tucker GT, Rostami-Hodjegan A (2004) Mechanism-based inactivation of cyp2d6 by methylenedioxymethamphetamine. Drug Metab Dispos 32:1213–1217
Farré M, Torre R, Mathúna B, Roset PN, Peiró AM, Torrens M, Ortuño J, Pujadas M, Camí J (2004) Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology 173:364–375
Segura M, Farre M, Pichini S, Peiro AM, Roset PN, Ramirez A, Ortuno J, Pacifici R, Zuccaro P, Segura J, de la Torre R (2005) Contribution of cytochrome p450 2d6 to 3,4-methylenedioxymethamphetamine disposition in humans: use of paroxetine as a metabolic inhibitor probe. Clin Pharmacokinet 44:649–660
Colado M, Williams J, Green A (1995) The hyperthermic and neurotoxic effects of “ecstasy” (MDMA) and 3,4 methylenedioxyamphetamine (MDA) in the dark agouti (DA) rat, a model of the cyp2d6 poor metabolizer phenotype. Br J Pharmacol 115:1281–1289
Koenig J, Lazarus C, Jeltsch H, Ben Hamida S, Riegert C, Kelche C, Jones BC, Cassel J-C (2005) MDMA (ecstasy) effects in pubescent rats: males are more sensitive than females. Pharmacol Biochem Behav 81:635–644
Fonsart J, Menet M-C, Declèves X, Galons H, Crété D, Debray M, Scherrmann J-M, Noble F (2008) Sprague–Dawley rats display metabolism-mediated sex differences in the acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy). Toxicol Appl Pharmacol 230:117–125
Fallon JK, Kicman AT, Henry JA et al (1999) Stereospecific analysis and enantiomeric disposition of 3,4-methylenedioxymethamphetamine (ecstasy) in humans. Clin Chem 45:1058–1069
Pizarro N, Farre M, Pujadas M, Peiro AM, Roset PN, Joglar J, de la Torre R (2004) Stereochemical analysis of 3,4-methylenedioxymethamphetamine and its main metabolites in human samples including the catechol-type metabolite (3,4-dihydroxymethamphetamine). Drug Metab Dispos 32:1001–1007
Meyer MR, Peters FT, Maurer HH (2008) The role of human hepatic cytochrome p450 isozymes in the metabolism of racemic MDMA and its enantiomers. Drug Metab Dispos 36:2345–2354
Kreth K, Kovar K, Schwab M, Zanger UM (2000) Identification of the human cytochromes p450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol 59:1563–1571
Maurer HH, Bickeboeller-Friedrich J, Kraemer T, Peters FT (2000) Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (“ecstasy”). Toxicol Lett 113:133–142
Segura M, Ortuño J, Farré M, McLure JA, Pujadas M, Pizarro N, Llebaria A, Joglar J, Roset PN, Segura J, de la Torre R (2001) 3,4-Dihydroxymethamphetamine (HHMA). A major in vivo 3,4-methylenedioxymethamphetamine (MDMA) metabolite in humans. Chem Res Toxicol 14:1203–1208
de la Torre R, Farre M, Mathuna BO, Roset PN, Pizarro N, Segura M, Torrens M, Ortuno J, Pujadas M, Cami J (2005) MDMA (ecstasy) pharmacokinetics in a cyp2d6 poor metaboliser and in nine cyp2d6 extensive metabolisers. Eur J Clin Pharmacol 61:551–554
Hiramatsu M, Kumagai Y, Unger SE, Cho AK (1990) Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther 254:521–527
Carvalho M, Hawksworth G, Milhazes N, Borges F, Monks TJ, Fernandes E, Carvalho F, Bastos ML (2002) Role of metabolites in MDMA (ecstasy)-induced nephrotoxicity: an in vitro study using rat and human renal proximal tubular cells. Arch Toxicol 76:581–588
Carvalho M, Remiao F, Milhazes N, Borges F, Fernandes E, Carvalho F, Bastos ML (2004) The toxicity of N-methyl-alpha-methyldopamine to freshly isolated rat hepatocytes is prevented by ascorbic acid and N-acetylcysteine. Toxicology 200:193–203
Carvalho M, Remião F, Milhazes N, Borges F, Fernandes E, Monteiro MC, Gonçalves MJ, Seabra V, Amado F, Carvalho F, Bastos ML (2004) Metabolism is required for the expression of ecstasy-induced cardiotoxicity in vitro. Chem Res Toxicol 17:623–632
Capela JP, Meisel A, Abreu AR, Branco PS, Ferreira LM, Lobo AM, Remião F, Bastos ML, Carvalho F (2006) Neurotoxicity of ecstasy metabolites in rat cortical neurons, and influence of hyperthermia. J Pharmacol Exp Ther 316:53–61
Capela JP, Macedo C, Branco PS, Ferreira LM, Lobo AM, Fernandes E, Remiao F, Bastos ML, Dirnagl U, Meisel A, Carvalho F (2007) Neurotoxicity mechanisms of thioether ecstasy metabolites. Neuroscience 146:1743–1757
Jones DC, Duvauchelle C, Ikegami A, Olsen CM, Lau SS, de la Torre R, Monks TJ (2005) Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J Pharmacol Exp Ther 313:422–431
Meisel C, Gerloff T, Kirchheiner J, Mrozikiewicz PM, Niewinski P, Brockmoller J, Roots I (2003) Implications of pharmacogenetics for individualizing drug treatment and for study design. J Mol Med 81:154–167
Johne A, Kopke K, Gerloff T, Mai I, Rietbrock S, Meisel C, Hoffmeyer S, Kerb R, Fromm MF, Brinkmann U, Eichelbaum M, Brockmoller J, Cascorbi I, Roots I (2002) Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther 72:584–594
Evans WE, McLeod HL (2003) Pharmacogenomics—drug disposition, drug targets, and side effects. N Engl J Med 348:538–549
Evans WE, Relling MV (1999) Pharmacogenomics: translating functional genomics into rational therapeutics. Science 286:487–491
Anzenbacher P, Anzenbacherová E (2001) Cytochromes p450 and metabolism of xenobiotics. Cell Mol Life Sci 58:737–747
Ingelman-Sundberg M, Oscarson M, McLellan RA (1999) Polymorphic human cytochrome p450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci 20:342–349
Tyndale R, Aoyama T, Broly F, Matsunaga T, Inaba T, Kalow W, Gelboin HV, Meyer UA, Gonzalez FJ (1991) Identification of a new variant cyp2d6 allele lacking the codon encoding lys-281: possible association with the poor metabolizer phenotype. Pharmacogenetics 1:26–32
Johansson I, Oscarson M, Yue QY, Bertilsson L, Sjoqvist F, Ingelman-Sundberg M (1994) Genetic analysis of the Chinese cytochrome p4502d locus: characterization of variant cyp2d6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol 46:452–459
Raimundo S, Fischer J, Eichelbaum M, Griese EU, Schwab M, Zanger UM (2000) Elucidation of the genetic basis of the common ‘intermediate metabolizer’ phenotype for drug oxidation by cyp2d6. Pharmacogenetics 10:577–581
Ramamoorthy Y, Yu A, Suh N, Haining RL, Tyndale RF, Sellers EM (2002) Reduced (±)-3,4-methylenedioxymethamphetamine (“ecstasy”) metabolism with cytochrome p450 2d6 inhibitors and pharmacogenetic variants in vitro. Biochem Pharmacol 63:2111–2119
Sachse C, Brockmoller J, Bauer S, Roots I (1997) Cytochrome p450 2d6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 60:284–295
Masimirembwa C, Persson I, Bertilsson L, Hasler J, Ingelman-Sundberg M (1996) A novel mutant variant of the cyp2d6 gene (cyp2d6*17) common in a black African population: association with diminished debrisoquine hydroxylase activity. Br J Clin Pharmacol 42:713–719
Aklillu E, Persson I, Bertilsson L, Johansson I, Rodrigues F, Ingelman-Sundberg M (1996) Frequent distribution of ultrarapid metabolizers of debrisoquine in an Ethiopian population carrying duplicated and multiduplicated functional cyp2d6 alleles. J Pharmacol Exp Ther 278:441–446
Ingelman-Sundberg M (2004) Pharmacogenetics of cytochrome p450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci 25:193–200
Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY, Hiratsuka A, Schmitz DA, Chu TY (1994) The demethylenation of methylenedioxymethamphetamine (“ecstasy”) by debrisoquine hydroxylase (cyp2d6). Biochem Pharmacol 47:1151–1156
Ramamoorthy Y, Tyndale RF, Sellers EM (2001) Cytochrome p450 2d6.1 and cytochrome p450 2d6.10 differ in catalytic activity for multiple substrates. Pharmacogenetics 11:477–487
Easton N, Fry J, O'Shea E, Watkins A, Kingston S, Marsden CA (2003) Synthesis, in vitro formation, and behavioural effects of glutathione regioisomers of alpha-methyldopamine with relevance to MDA and MDMA (ecstasy). Brain Res 987:144–154
de la Torre R, Farre M (2004) Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci 25:505–508
Gilhooly TC, Daly AK (2002) Cyp2d6 deficiency, a factor in ecstasy related deaths. Br J Clin Pharmacol 54:69–70
O’Donohoe A, O’Flynn K, Shields K, Hawi Z, Gill M (1998) MDMA toxicity: no evidence for a major influence of metabolic genotype at CYP2D6. Addict Biol 3:309–314
Schwab M, Seyringer E, Brauer RB, Hellinger A, Griese EU (1999) Fatal MDMA intoxication. Lancet 353:593–594
Lima JJ, Thomason DB, Mohamed MH, Eberle LV, Self TH, Johnson JA (1999) Impact of genetic polymorphisms of the beta2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther 65:519–525
Zanger UM, Raimundo S, Eichelbaum M (2004) Cytochrome p450 2d6: overview and update on pharmacology, genetics, biochemistry. Naunyn-Schmiedebergs Arch Pharmacol 369:23–37
Krebsfaenger N, Murdter TE, Zanger UM, Eichelbaum MF, Doehmer J (2003) V79 Chinese hamster cells genetically engineered for polymorphic cytochrome p450 2d6 and their predictive value for humans. ALTEX 20:143–154
Carmo H, Brulport M, Hermes M, Oesch F, Silva R, Ferreira LM, Branco PS, Boer DD, Remiao F, Carvalho F, Schon MR, Krebsfaenger N, Doehmer J, Bastos MD, Hengstler JG (2006) Influence of cyp2d6 polymorphism on 3,4-methylenedioxymethamphetamine (“ecstasy”) cytotoxicity. Pharmacogenet Genomics 16:789–799
Carmo H, Brulport M, Hermes M, Oesch F, deBoer D, Remião F, Carvalho F, Schön MR, Krebsfaenger N, Doehmer J, Bastos ML, Hengstler JG (2007) Cyp2d6 increases toxicity of the designer drug 4-methylthioamphetamine (4-MTA). Toxicology 229:236–244
Carvalho M, Carvalho F, Bastos ML (2001) Is hyperthermia the triggering factor for hepatotoxicity induced by 3,4-methylenedioxymethamphetamine (ecstasy)? An in vitro study using freshly isolated mouse hepatocytes. Arch Toxicol 74:789–793
Carmo H, Boer DD, Remiao F, Carvalho F, Reys LAD, Bastos ML (2004) Metabolism of the designer drug 4-bromo-2,5-dimethoxyphenethylamine (2c-b) in mice, after acute administration. J Chromatogr B Analyt Technol Biomed Life Sci 811:143–152
Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM (1996) Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6:243–250
Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J (1995) Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34:4202–4210
Lundstrom K, Tenhunen J, Tilgmann C, Karhunen T, Panula P, Ulmanen I (1995) Cloning, expression and structure of catechol-O-methyltransferase. Biochim Biophys Acta 1251:1–10
Weinshilboum RM, Otterness DM, Szumlanski CL (1999) Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol 39:19–52
Zhu BT (2002) Catechol-O-methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab 3:321–349
Ameyaw MM, Syvanen AC, Ulmanen I, Ofori-Adjei D, McLeod HL (2000) Pharmacogenetics of catechol-O-methyltransferase: frequency of low activity allele in a Ghanaian population. Hum Mutat 16:445–446
McLeod HL, Fang L, Luo X, Scott EP, Evans WE (1994) Ethnic differences in erythrocyte catechol-O-methyltransferase activity in black and white Americans. J Pharmacol Exp Ther 270:26–29
McLeod HL, Collie-Duguid ES, Vreken P, Johnson MR, Wei X, Sapone A, Diasio RB, Fernandez-Salguero P, vanKuilenberg AB, vanGennip AH, Gonzalez FJ (1998) Nomenclature for human DPYD alleles. Pharmacogenetics 8:455–459
Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR (2003) Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A 100:6186–6191
Freimuth RR, Eckloff B, Wieben ED, Weinshilboum RM (2001) Human sulfotransferase sult1c1 pharmacogenetics: gene resequencing and functional genomic studies. Pharmacogenetics 11:747–756
Nagata K, Yamazoe Y (2000) Pharmacogenetics of sulfotransferase. Annu Rev Pharmacol Toxicol 40:159–176
Daly AK (2003) Pharmacogenetics of the major polymorphic metabolizing enzymes. Fundam Clin Pharmacol 17:27–41
Monaghan G, Ryan M, Seddon R, Hume R, Burchell B (1996) Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 347:578–581
Aono S, Adachi Y, Uyama E, Yamada Y, Keino H, Nanno T, Koiwai O, Sato H (1995) Analysis of genes for bilirubin UDP-glucuronosyltransferase in Gilbert’s syndrome. Lancet 345:958–959
deMorais SM, Uetrecht JP, Wells PG (1992) Decreased glucuronidation and increased bioactivation of acetaminophen in Gilbert’s syndrome. Gastroenterology 102:577–586
Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ (1998) Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 101:847–854
Miners JO, McKinnon RA, Mackenzie PI (2002) Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology 181–182:453–456
Zheng Z, Park JY, Guillemette C, Schantz SP, Lazarus P (2001) Tobacco carcinogen-detoxifying enzyme UGT1a7 and its association with orolaryngeal cancer risk. J Natl Cancer Inst 93:1411–1418
Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB (1994) Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J 300:271–276
Seidegard J, Vorachek WR, Pero RW, Pearson WR (1988) Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A 85:7293–7297
Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J (1997) Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem 272:10004–10012
McLellan RA, Oscarson M, Alexandrie AK, Seidegard J, Evans DA, Rannug A, Ingelman-Sundberg M (1997) Characterization of a human glutathione S-transferase mu cluster containing a duplicated GSTM1 gene that causes ultrarapid enzyme activity. Mol Pharmacol 52:958–965
Hashimoto T, Hashimoto K, Matsuzawa D, Shimizu E, Sekine Y, Inada T, Ozaki N, IIwata N, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Iyo M (2005) A functional glutathione S-transferase P1 gene polymorphism is associated with methamphetamine-induced psychosis in Japanese population. Am J Med Genet B Neuropsychiatr Genet 135:5–9
Mueller M, Peters F, Maurer H, McCann U, Ricaurte GA (2008) Non-linear pharmacokinetics of MDMA (“ecstasy”) and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses. J Pharmacol Exp Ther 327:38–44
Bowyer JF, Young JF, Slikker JW, Itzak Y, Mayorga AJ, Newport GD, Ali SF, Frederick DL, Paule MG (2003) Plasma levels of parent compound and metabolites after doses of either d-fenfluramine or d-3,4-methylenedioxymethamphetamine (MDMA) that produce long-term serotonergic alterations. Neurotoxicology 24:379–390
Lim HK, Su Z, Foltz RL (1993) Stereoselective disposition: enantioselective quantitation of 3,4-(methylenedioxy) methamphetamine and three of its metabolites by gas chromatography/electron capture negative ion chemical ionization mass spectrometry. Biol Mass Spectrom 22:403–411
Carvalho M, Carvalho F, Pereira M, Pires-das-Neves R, Bastos M (2002) Effect of 3,4-methylenedioxymethamphetamine (“ecstasy”) on body temperature and liver antioxidant status in mice. Influence of ambient temperature. Arch Toxicol 76:166–172
Miller DB, O’Callaghan JP (1995) The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol 11:177–192
Johnson EA, Sharp DS, Miller DB (2000) Restraint as a stressor in mice: against the dopaminergic neurotoxicity of d-MDMA, low body weight mitigates restraint-induced hypothermia and consequent neuroprotection. Brain Res 875:107–118
Colado MI, Camarero J, Mechan AO, Sanchez V, Esteban B, Elliott JM, Green AR (2001) A study of the mechanisms involved in the neurotoxic action of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) on dopamine neurones in mouse brain. Br J Pharmacol 134:1711–1723
Fantegrossi WE, Kiessel CL, De la Garza R, Woods JH (2005) Serotonin synthesis inhibition reveals distinct mechanisms of action for MDMA and its enantiomers in the mouse. Psychopharmacology 181:529–536
Bexis S, Docherty JR (2005) Role of [alpha]2a-adrenoceptors in the effects of MDMA on body temperature in the mouse. Br J Pharmacol 146:1–6
Mills EM, Banks ML, Sprague JE, Finkel T (2003) Uncoupling the agony from ecstasy. Nature 426:403–404
Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH (2003) Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology 166:202–211
Pontes H, Duarte JA, de Pinho PG, Soares ME, Fernandes E, Dinis-Oliveira RJ, Sousa C, Silva R, Carmo H, Casal S, Remião F, Carvalho F, Bastos ML (2008) Chronic exposure to ethanol exacerbates MDMA-induced hyperthermia and exposes liver to severe MDMA-induced toxicity in CD1 mice. Toxicology 252:64–71
Broening HW, Bowyer JF, Slikker WJ (1995) Age dependent sensitivity of rats to the long term effects of the serotonergic neurotoxicant (±)-3,4methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther 275:325–333
Malberg J, Seiden L (1998) Small changes in ambient temperature cause large changes in 3,4-methylenodioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 18:5086–5094
Green AR, O’Shea E, Saadat KS, Elliott JM, Colado MI (2005) Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol 146:306–312
Gordon CJ, Watkinson WP, O'Callaghan JP, Miller DB (1991) Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav 38:339–344
Nash JF Jr., Meltzer HY, Gudelsky GA (1988) Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther 245:873–879
Schmidt C, Abbate G, Black C, Taylor V (1990) Selective 5-hydroxytryptamine2 receptor antagonists protect against the neurotoxicity of methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther 255:478–483
Mechan AO, Esteban B, O’Shea E, Elliot JM, Colado MI, Green AR (2002) The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA “ecstasy”) to rats. Br J Pharmacol 135:170–180
Malberg JE, Sabol KE, Seiden LS (1996) Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. J Pharmacol Exp Ther 278:258–267
Mechan AO, O’Shea E, Elliot JM, Colado MI, Green AR (2001) A neurotoxic dose of 3, 4-methylenedioxymethamphetamine (MDMA; ecstasy) to rats results in a long-term defect in thermoregulation. Psychopharmacology 155:413–418
Sanchez V, O'Shea E, Saadat KS, Elliott JM, Colado MI, Green AR (2004) Effect of repeated (“binge”) dosing of MDMA to rats housed at normal and high temperature on neurotoxic damage to cerebral 5-HT and dopamine neurones. J Psychopharmacol 18:412–416
Walker Q, Williams C, Jotwani R, Waller S, Francis R, Kuhn C (2007) Sex differences in the neurochemical and functional effects of MDMA in Sprague–Dawley rats. Psychopharmacology 189:435–445
Goni-Allo B, Ó Mathúna B, Segura M, Puerta E, Lasheras B, de la Torre R, Aguirre N (2008) The relationship between core body temperature and 3,4-methylenedioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology 197:263–278
Shioda K, Nisijima K, Yoshino T, Kuboshima K, Iwamura T, Yui K, Kato S (2008) Risperidone attenuates and reverses hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neurotoxicology 29:1030–1036
Fernandez F, Aguerre S, Mormède P, Chaouloff F (2002) Influences of the corticotropic axis and sympathetic activity on neurochemical consequences of 3,4-methylenedioxymethamphetamine (MDMA) administration in Fischer 344 rats. Eur J Neurosci 16:607–618
Sprague JE, Banks ML, Cook VJ, Mills EM (2003) Hypothalamic–pituitary–thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy). J Pharmacol Exp Ther 305:159–166
Blessing W, Seaman B (2003) 5-Hidroxitriptamine2a receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience 117:939–948
Blessing WW, Seaman B, Pedersen NP, Ootsuka Y (2003) Clozapine reverses hyperthermia and sympathetically mediated cutaneous vasoconstriction induced by 3,4-methylenedioxymethamphetamine (ecstasy) in rabbits and rats. J Neurosci 23:6385–6391
Saadat KS, Elliott JM, Colado MI, Green AR (2004) Hyperthermic and neurotoxic effect of 3,4-methylenedioxymethamphetamine (MDMA) in guinea pigs. Psychopharmacology 173:452–453
Fiege M, Wappler F, Weisshorn R, Gerbershagen MU, Menge M, Schulte Esch J (2003) Induction of malignant hyperthermia in susceptible swine by 3,4-methylenedioxymethamphetamine (“ecstasy”). Anesthesiology 99:1132–1136
Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA (2006) Effects of (±)3,4-methylenedioxymethamphetamine, (±)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience 142:515–525
Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA (2006) Impact of ambient temperature on hyperthermia induced by (±)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology 32:673–681
Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN (2006) Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys. Drug Alcohol Depend 82:276–281
Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA (2007) Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos 35:1840–1845
Miller DB, O’Callaghan JP (1994) Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:752–760
Sanchez V, Camarero J, Esteban B, Peter MJ, Green AR, Colado MI (2001) The mechanisms involved in the long-lasting neuroprotective effect of fluoxetine against MDMA (“ecstasy”)-induced degeneration of 5-HT nerve endings in rat brain. Br J Pharmacol 134:46–57
Schmidt CJ, Taylor VL (1990) Reversal of the acute effects of 3,4-methylenedioxymethamphetamine by 5-HT uptake inhibitors. Eur J Pharmacol 181:133–136
Sugimoto Y, Okhura M, Inoue K, Yamada J (2001) Involvement of serotonergic and dopaminergic mechanisms in hyperthermia induced by a serotonin-releasing drug, p-chloroamphetamine in mice. Eur J Pharmacol 430:265–268
Ootsuka Y, Nalivaiko E, Blessing WW (2004) Spinal 5-HT2a receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats: relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, “ecstasy”) and its reversal by clozapine. Brain Res 1014:34–44
Sprague JE, Brutcher RE, Mills EM, Caden D, Rusyniak DE (2004) Attenuation of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-induced rhabdomyolysis with α1- plus β3-adrenoreceptor antagonists. Br J Pharmacol 142:667–670
Sprague JE, Moze P, Caden D, Rusyniak DE, Holmes C, Goldstein DS, Mills EM (2005) Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) in an animal model. Crit Care Med 33:1311–1316
Bexis S, Docherty JS (2008) Role of α1-adrenoceptor subtypes in the effects of methylenedioxy methamphetamine (MDMA) on body temperature in the mouse. Br J Pharmacol 153:591–597
Wyeth RP, Mills EM, Ullman A, Kenaston MA, Burwell J, Sprague JE (2009) The hyperthermia mediated by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) is sensitive to sex differences. Toxicol Appl Pharmacol 235:33–38
Henry J (1992) Ecstasy and the dance of death. Br Med J 350:5–6
McNamara MG, Kelly JP, Leonard BE (1995) Some behavioural and neurochemical aspects of subacute (6)3,4-methylenedioxymethamphetamine administration in rats. Pharmacol Biochem Behav 52:479–484
De Souza I, Kelly JP, Harkin AJ, Leonard BE (1997) An appraisal of the pharmacological and toxicological effects of a single oral administration of 3,4-methylenedioxymethamphetamine (MDMA) in the rat. Pharmacol Toxicol 80:207–210
Shankaran M, Gudelsky GA (1999) A neurotoxic regimen of MDMA suppresses behavioral, thermal and neurochemical responses to subsequent MDMA administration. Psychopharmacology 147:66–72
Spanos LJ, Yamamoto BK (1989) Acute and subchronic effects of methylenodioxymethamphetamine [(±)MDMA] on locomotion and serotonin syndrome behavior in the rat. Pharmacol Biochem Behav 32:835–840
Piper BJ, Fraiman JB, Meyer JS (2005) Repeated MDMA (“ecstasy”) exposure in adolescent male rats alters temperature regulation, spontaneous motor activity, attention, and serotonin transporter binding. Developmental Psychobiology 47:145–157
Morley KC, Arnold JC, McGregor IS (2005) Serotonin (1a) receptor involvement in acute 3,4-methylenedioxymethamphetamine (MDMA) facilitation of social interaction in the rat. Prog Neuropsychopharmacol Biol Psychiatry 29:648–657
Fitzgerald JL, Reid JJ (1994) Sympathomimetic actions of methylenedioxymethamphetamine in rat and rabbit isolated cardiovascular tissues. J Pharm Pharmacol 46:826–832
Jaehne EJ, Salem A, Irvine RJ (2008) The effect of long-term repeated exposure to 3,4-methylenedioxymethamphetamine on cardiovascular and thermoregulatory changes. Psychopharmacology 201:161–170
O’Cain PA, Hletko SB, Ogden BA, Varner KJ (2000) Cardiovascular and sympathetic responses and reflex changes elicited by MDMA. Physiol Behav 70:141–148
Badon LA, Hicks A, Lord K, Ogden BA, Meleg-Smith S, Varner KJ (2002) Changes in cardiovascular responsiveness and cardiotoxicity elicited during binge administration of ecstasy. J Pharmacol Exp Ther 302:898–907
Bexis S, Docherty JR (2006) Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve [alpha]-adrenoceptors. Br J Pharmacol 147:926–934
Lin HQ, Burden PM, Christie MJ, Johnston GAR (1999) The anxiogenic-like and anxiolytic-like effects of MDMA on mice in the elevated plus-maze: a comparison with amphetamine. Pharmacol Biochem Behav 62:403–408
Navarro JF, Maldonado E (2002) Acute and subchronic effects of MDMA (“ecstasy”) on anxiety in male mice tested in the elevated plus-maze. Prog Neuropsychopharmacol Biol Psychiatry 26:1151–1154
Sumnall HR, O'Shea E, Marsden CA, Cole JC (2004) The effects of MDMA pretreatment on the behavioural effects of other drugs of abuse in the rat elevated plus-maze test. Pharmacol Biochem Behav 77:805–814
Ho Y-J, Pawlak CR, Guo L, Schwarting RKW (2004) Acute and long-term consequences of single MDMA administration in relation to individual anxiety levels in the rat. Behav Brain Res 149:135–144
Bull EJ, Hutson PH, Fone KCF (2004) Decreased social behaviour following 3,4-methylenedioxymethamphetamine (MDMA) is accompanied by changes in 5-HT2a receptor responsivity. Neuropharmacology 46:202–210
Fone KC, Beckett SR, Topham IA, Swettenham J, Ball M, Maddocks L (2002) Long-term changes in social interaction and reward following repeated MDMA administration to adolescent rats without accompanying serotonergic neurotoxicity. Psychopharmacology 159:437–444
Poland RE (1990) Diminished corticotropin and enhanced prolactin responses to 8-hydroxy-2(di-n-propylamino)tetralin in methylenedioxymethamphetamine pretreated rats. Neuropharmacology 29:1099–1101
Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB (2008) Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience 152:773–784
Connor TJ (2004) Methylenedioxymethamphetamine (MDMA, ‘ecstasy’): a stressor on the immune system. Immunology 111:357–367
Connor TJ, McNamara MG, Finn D, Currid A, O’Malley M, Redmond AM, Kelly JP, Leonard BE (1998) Acute 3,4-methylenedioxymethamphetamine (MDMA) administration produces a rapid and sustained suppression of immune function in the rat. Immunopharmacology 38:253–260
Connor TJ, Kelly JP, McGee M, Leonard BE (2000) Methylenedioxymethamphetamine (MDMA; ecstasy) suppresses IL-1[beta] and TNF-[alpha] secretion following an in vivo lipopolysaccharide challenge. Life Sci 67:1601–1612
Nelson DA, Nirmaier JL, Singh SJ, Tolbert MD, Bost KL (2008) Ecstasy (3,4-methylenedioxymethamphetamine) limits murine gammaherpesvirus-68 induced monokine expression. Brain Behav Immun 22:912–922
Schenk S, Gittings D, Johnstone M, Daniela E (2003) Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology 169:21–27
Schenk S, Hely L, Lake B, Daniela E, Gittings D, Mash DC (2007) MDMA self-administration in rats: acquisition, progressive ratio responding and serotonin transporter binding. Eur J Neurosci 26:3229–3236
Wakonigg G, Sturm K, Saria A, Zernig G (2003) Methylenedioxymethamphetamine (MDMA, ‘ecstasy’) serves as a robust positive reinforcer in a rat runway procedure. Pharmacology 69:180–182
Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch K-P, Robledo P, Maldonado R (2007) 3,4-Methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol Psychiatry 62:669–679
Touriño C, Ledent C, Maldonado R, Valverde O (2008) CB1 cannabinoid receptor modulates 3,4-methylenedioxymethamphetamine acute responses and reinforcement. Biol Psychiatry 63:1030–1038
Lile JA, Ross JT, Nader MA (2005) A comparison of the reinforcing efficacy of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) with cocaine in rhesus monkeys. Drug Alcohol Depend 78:135–140
Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G (2002) 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology 161:356–364
Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G (2004) Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology 29:1270–1281
Steketee JD (2003) Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev 41:203–228
Vanderschuren LJMJ, Schmidt ED, De Vries TJ, Van Moorsel CAP, Tilders FJH, Schoffelmeer ANM (1999) A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci 19:9579–9586
Ramos M, Goni-Allo B, Aguirre N (2005) Administration of SCH 23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: an effect mediated by 5-HT2c receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology 30:2180–2191
Kalivas PW, Duffy P, White SR (1998) MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology 18:469–479
Colussi-Mas J, Schenk S (2008) Acute and sensitized response to 3,4-methylenedioxymethamphetamine in rats: different behavioral profiles reflected in different patterns of Fos expression. Eur J Neurosci 28:1895–1910
Hall AP, Henry JA (2006) Acute toxic effects of ‘ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth 96:678–685
Vollenweider FX, Gamma A, Liechti ME, Huber T (1998) Psychological and cardiovascular effects and short-term sequelae of MDMA (“ecstasy”) in MDMA-naive healthy volunteers. Neuropsychopharmacology 19:241–251
Cohen RS, Cocores J (1997) Neuropsychiatric manifestations following the use of 3,4-methylenedioxymethamphetamine (MDMA: “ecstasy”). Prog Neuropsychopharmacol Biol Psychiatry 21:727–734
Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX (2000) Psychological and physiological effects of MDMA (“ecstasy”) after pre-treatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology 23:396–404
Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA (2008) Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol 28:432–440
Tancer M, Johanson C-E (2007) The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 189:565–573
Farré M, Abanades S, Roset PN, Peiro AM, Torrens M, O’Mathuna B, Segura M, de la Torre R (2007) Pharmacological interaction between 3,4-methylenedioxymethamphetamine (ecstasy) and paroxetine: pharmacological effects and pharmacokinetics. J Pharmacol Exp Ther 323:954–962
Tancer M, Johanson CE (2003) Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend 72:33–44
Freedman RR, Johanson CE, Tancer ME (2005) Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 183:248–256
Liechti ME, Vollenweider FX (2000) The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3,4-methylenedioxymethamphetamine (‘ecstasy’) in healthy volunteers. J Psychopharmacol 14:269–274
Irvine RJ, Keane M, Felgate P, McCann UD, Callaghan PD, White JM (2005) Plasma drug concentrations and physiological measures in “dance party” participants. Neuropsychopharmacology 31:424–430
Kunitz O, Ince A, Kuhlen R, Rossaint R (2003) Hyperpyrexia and rhabdomyolysis after ecstasy (MDMA) intoxication. Anaesthesist 52:511–515
Mallick A, Bodenham AR (1997) MDMA induced hyperthermia: a survivor with an initial body temperature of 42.9 degrees C. J Accid Emerg Med 14:336–338
Connolly E, O'Callaghan G (1999) MDMA toxicity presenting with severe hyperpyrexia: a case report. Crit Care Resusc 1:368–370
Henry JA, Fallon JK, Kicman AT, Hutt AJ, Cowan DA, Forsling M (1998) Low-dose MDMA (“ecstasy”) induces vasopressin secretion. Lancet 351:1784
Wolff K, Tsapakis EM, Winstock AR, Hartley D, Holt D, Forsling ML, Aitchison KJ (2006) Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. J Psychopharmacol 20:400–410
Gerra G, Zaimovic A, Franchini D, Palladino M, Guicastro G, Reali N, Maestri D, Caccavari R, Delsignore R, Brambilla F (1998) Neuroendocrine responses of healthy volunteers to “techno-music”: relationships with personality traits and emotional state. Int J Psychophysiol 28:99–111
Pacifici R, Zuccaro P, Farré M, Pichini S, Di Carlo S, Roset PN, Ortuño J, Pujadas M, Bacosi A, Menoyo E, Segura J, de la Torre R (2001) Effects of repeated doses of MDMA (“ecstasy”) on cell-mediated immune response in humans. Life Sci 69:2931–2941
Klitzman RL, Pope HG Jr., Hudson JI (2000) MDMA (“ecstasy”) abuse and high-risk sexual behaviors among 169 gay and bisexual men. Am J Psychiatry 157:1162–1164
Davison D, Parrott AC (1997) Ecstasy (MDMA) in recreational users: self-reported psychological and physiological effects. Hum Psychopharmacol 12:221–226
McCann UD, Slate SO, Ricaurte GA (1996) Adverse reactions with 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”). Drug Saf 15:107–115
Liechti ME, Kunz I, Kupferschmidt H (2005) Acute medical problems due to ecstasy use. Case-series of emergency department visits. Swiss Med Wkly 135:652–627
Milroy CM, Clark JC, Forrest AR (1996) Pathology of deaths associated with “ecstasy” and “Eve” misuse. J Clin Pathol 49:149–153
Logan SC, Stickle B, O’Keefe N, Hewitson H (1993) Survival following “ecstasy” ingestion with a peak temperature of 42°C. Anaesthesia 48:1017–1018
Garcia-Repetto R, Moreno E, Soriano T, Jurado C, Gimenez MP, Menendez M (2003) Tissue concentrations of MDMA and its metabolite MDA in three fatal cases of overdose. Forensic Sci Int 135:110–114
SAMHDA. (2002) Emergency department trends from the drug abuse warning network, final estimates 1994–2001. In DAWN Series D-21 (02-3635, D. P. N. S., Ed.), Substance Abuse and Mental Health Services Administration Office of Applied Studies, Rockville, MD
Gore SM (1999) Fatal uncertainty: death rate from use of ecstasy or heroin. Lancet 354:1265–1266
Schmidt C, Wu L, Lovenberg W (1986) Methylenedioxymethamphetamine: a potentially neurotoxic amphetamine analogue. Eur J Pharmacol 124:175–178
Schmidt CJ (1987) Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther 240:1–7
Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS (1987) Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther 241:338–345
Stone DM, Johnson M, Hanson GR, Gibb JW (1987) A comparison of the neurotoxic potential of methylenedioxyamphetamine (MDA) and its N-methylated and N-ethylated derivatives. Eur J Pharmacol 134:245–248
Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB (1987) 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther 242:911–916
Stone DM, Hanson GR, Gibb JW (1987) Differences in the central serotonergic effects of methylenedioxymethamphetamine (MDMA) in mice and rats. Neuropharmacology 26:1657–1661
Ricaurte GA, Bryan G, Strauss L, Seiden LS, Schuster CR (1985) Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science 229:986–988
Warren M, Larner S, Kobeissy F, Brezing C, Jeung J, Hayes R, Gold M, Wang K (2007) Calpain and caspase proteolytic markers co-localize with rat cortical neurons after exposure to methamphetamine and MDMA. Acta Neuropathologica 114:277–286
Xie T, Tong L, McLane MW, Hatzidimitriou G, Yuan J, McCann U, Ricaurte GA (2006) Loss of serotonin transporter protein after MDMA and other ring-substituted amphetamines. Neuropsychopharmacology 31:2639–2651
Yeh S (1999) N-tert-butyl-alpha-phenylnitrone protects against 3,4-methylenedioxymethamphetamine-induced depletion of serotonin in rats. Synapse 31:169–177
Sprague JE, Nichols DE (1995) Inhibition of MAO-B protects against MDMA-induced neurotoxicity in the striatum. Psychopharmacology 118:357–359
Shankaran M, Yamamoto BK, Gudelsky GA (1999) Involvement of the SERT in the formation of hydroxyl radicals induced by 3,4-methylenedioxymethamphetamine. Eur J Pharmacol 385:103–110
Schmued L (2003) Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res 974:127–133
O’Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME (1988) Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci 8:2788–2803
Molliver ME, Berger UV, Mamounas LA, Molliver DC, O’Hearn E, Wilson MA (1990) Neurotoxicity of MDMA and related compounds: anatomic studies. Ann N Y Acad Sci 600:649–664
Fischer C, Hatzidimitriou G, Wlos J, Katz J, Ricaurte G (1995) Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (±)3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). J Neurosci 15:5476–5485
Colado MI, Murray TK, Green AR (1993) 5-HT loss in rat brain following 3,4 methylenedioxymethamphetamine (MDMA), p-chloroamphetamine and fenfluramine administration and effects of chlormethiazole and dizocilpine. Br J Pharmacol 108:583–589
Armstrong BD, Noguchi KK (2004) The neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine on serotonin, dopamine, and GABA-ergic terminals: an in-vitro autoradiographic study in rats. Neurotoxicology 25:905–914
Aguirre N, Barrionuevo M, Lasheras B, Del Rio J (1998) The role of dopaminergic systems in the perinatal sensitivity to 3,4-methylenedioxymethamphetamine-induced neurotoxicity in rats. J Pharmacol Exp Ther 286:1159–1165
O'Shea E, Orio L, Escobedo I, Sanchez V, Camarero J, Green AR, Colado MI (2006) MDMA-induced neurotoxicity: long-term effects on 5-HT biosynthesis and the influence of ambient temperature. Br J Pharmacol 148:778–785
Izco M, Orio L, O’Shea E, Colado M (2007) Binge ethanol administration enhances the MDMA-induced long-term 5-HT neurotoxicity in rat brain. Psychopharmacology 189:459–470
McNamara R, Kerans A, O’Neill B, Harkin A (2006) Caffeine promotes hyperthermia and serotonergic loss following co-administration of the substituted amphetamines, MDMA (“ecstasy”) and MDA (“love”). Neuropharmacology 50:69–80
Hewitt K, Green A (1994) Chlormethiazole, dizocilpine and haloperidol prevent the degeneration of serotonergic nerve terminals induced by administration of MDMA (“ecstasy”) to rats. Neuropharmacology 33:1589–1595
Murray TK, Williams JL, Misra A, Colado MI, Green AR (1997) The spin trap reagent PBN attenuates degeneration of 5-HT neurones in rat brain induced by p-chloroamphetamine but not fenfluramine. Neuropharmacology 35:1615–1620
Chipana C, Camarasa J, Pubill D, Escubedo E (2008) Memantine prevents MDMA-induced neurotoxicity. Neurotoxicology 29:179–183
O'Shea E, Granados R, Esteban B, Colado MI, Green AR (1998) The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (“ecstasy”). Neuropharmacology 37:919–926
Hervías I, Lasheras B, Aguirre N (2000) 2-Deoxy-d-glucose prevents and nicotinamide potentiates 3,4-methylenedioxymethamphetamine-induced serotonin neurotoxicity. J Neurochem 75:982–990
Bull EJ, Hutson PH, Fone KCF (2003) Reduced social interaction following 3,4-methylenedioxymethamphetamine is not associated with enhanced 5-HT2c receptor responsivity. Neuropharmacology 44:439–448
Schmidt CJ, Taylor VL (1987) Depression of rat brain tryptophan hydroxylase activity following the acute administration of methylenedioxymethamphetamine. Biochem Pharmacol 36:4095–4102
Stone DM, Stahl DC, Hanson GR, Gibb JW (1986) The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur J Pharmacol 128:41–48
Battaglia G, Yeh SY, De Souza EB (1988) MDMA-induced neurotoxicity: parameters of degeneration and recovery of brain serotonin neurons. Pharmacol Biochem Behav 29:269–274
Johnson M, Letter AA, Merchant K, Hanson GR, Gibb JW (1988) Effects of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine isomers on central serotonergic, dopaminergic and nigral neurotensin systems of the rat. J Pharmacol Exp Ther 244:977–982
Capela JP, Lautenschlager M, Dirnagl U, Bastos ML, Carvalho F, Meisel A (2008) 5,7-Dihydroxitryptamine toxicity to serotonergic neurons in serum free raphe cultures. Eur J Pharmacol 588:232–238
Baumgarten HG, Bjorklund A (1976) Neurotoxic indoleamines and monoamine neurons. Annu Rev Pharmacol Toxicol 16:101–111
Colado MI, Green AR (1995) The spin trap reagent a-phenyl-N-tert-butyl nitrone prevents “ecstasy”-induced neurodegeneration of 5-hydroxytryptamine neurons. Eur J Pharmacol 280:343–346
Aguirre N, Ballaz S, Lasheras B, Del Río J (1998) MDMA (“ecstasy”) enhances 5-HT1a receptor density and 8-OH-DPAT-induced hypothermia: blockade by drugs preventing 5-hydroxytryptamine depletion. Eur J Pharmacol 346:181–188
Scanzello CR, Hatzidimitriou G, Martello AL, Katz JL, Ricaurte GA (1993) Serotonergic recovery after (±)3,4-(methylenedioxy) methamphetamine injury: observations in rats. J Pharmacol Exp Ther 264:1484–1491
Sabol KE, Lew R, Richards JB, Vosmer GL, Seiden LS (1996) Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52-week period. Part I: synaptosomal uptake and tissue concentrations. J Pharmacol Exp Ther 276:846–854
Lew R, Sabol KE, Chou C, Vosmer GL, Richards J, Seiden LS (1996) Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52-week period. Part II: radioligand binding and autoradiography studies. J Pharmacol Exp Ther 276:855–865
Kovács GG, Andó RD, Ádori C, Kirilly E, Benedek A, Palkovits M, Bagdy G (2007) Single dose of MDMA causes extensive decrement of serotoninergic fibre density without blockage of the fast axonal transport in dark agouti rat brain and spinal cord. Neuropathol Appl Neurobiol 33:193–203
Wang X, Baumann MH, Xu H, Morales M, Rothman RB (2005) (±)-3,4-Methylenedioxymethamphetamine administration to rats does not decrease levels of the serotonin transporter protein or alter its distribution between endosomes and the plasma membrane. J Pharmacol Exp Ther 314:1002–1012
Baumann MH, Wang X, Rothman RB (2007) 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology 189:407–424
Wang X, Baumann MH, Xu H, Rothman RB (2004) 3,4-Methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse 53:240–248
Wang X, Baumann MH, Dersch CM, Rothman RB (2007) Restoration of 3,4-methylenedioxymethamphetamine-induced 5-HT depletion by the administration of l-5-hydroxytryptophan. Neuroscience 148:212–220
Meyer JS, Grande M, Johnson K, Ali SF (2004) Neurotoxic effects of MDMA (“ecstasy”) administration to neonatal rats. Int J Dev Neurosci 22:261–271
Tamburini I, Blandini F, Gesi M, Frenzilli G, Nigro M, Giusiani M, Paparelli A, Fornai F (2006) MDMA induces caspase-3 activation in the limbic system but not in striatum. Ann N Y Acad Sci 1074:377–381
Jensen KF, Olin J, Haykal-Coates N, O’Callaghan J, Miller DB, de Olmos JS (1993) Mapping toxicant-induced nervous system damage with a cupric silver stain: a quantitative analysis of neural degeneration induced by 3,4-methylenedioxymethamphetamine. NIDA Res Monogr 136:133–149, discussion 150–154
Hernandez-Rabaza V, Dominguez-Escriba L, Barcia JA, Rosel JF, Romero FJ, Garcia-Verdugo JM, Canales JJ (2006) Binge administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) impairs the survival of neural precursors in adult rat dentate gyrus. Neuropharmacology 51:967–973
Ádori C, Andó RD, Kovács GG, Bagdy G (2006) Damage of serotonergic axons and immunolocalization of Hsp27, Hsp72, and Hsp90 molecular chaperones after a single dose of MDMA administration in dark agouti rat: temporal, spatial, and cellular patterns. J Comp Neurol 497:251–269
Stumm G, Schlegel J, Schafer T, Wurz C, Mennel H, Krieg J, Vedder H (1999) Amphetamines induce apoptosis and regulation of bcl-x splice variants in neocortical neurons. FASEB J 13:1065–1072
Jimenez A, Jorda EG, Verdaguer E, Pubill D, Sureda FX, Canudas AM, Escubedo E, Camarasa J, Camins A, Pallas M (2004) Neurotoxicity of amphetamine derivatives is mediated by caspase pathway activation in rat cerebellar granule cells. Toxicol Appl Pharmacol 196:223–234
Capela JP, Ruscher K, Lautenschlager M, Freyer D, Dirnagl U, Gaio AR, Bastos ML, Meisel A, Carvalho F (2006) Ecstasy-induced cell death in cortical neuronal cultures is serotonin 2a-receptor-dependent and potentiated under hyperthermia. Neuroscience 139:1069–1081
Capela JP, Fernandes E, Remiao F, Bastos ML, Meisel A, Carvalho F (2007) Ecstasy induces apoptosis via 5-HT2a-receptor stimulation in cortical neurons. Neurotoxicology 28:868–875
Aguirre N, Barrionuevo M, Ramirez MJ, Del Rio J, Lasheras B (1999) Alpha-lipoic acid prevents 3,4-methylenedioxymethamphetamine (MDMA)-induced neurotoxicity. NeuroReport 10:3675–3680
Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E (2003) Different glial response to methamphetamine and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn Schmiedebergs Arch Pharmacol 367:490–499
O’Callaghan JP, Miller DB (1994) Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:741–751
Orio L, O’Shea E, Sanchez V, Pradillo JM, Escobedo I, Camarero J, Moro MA, Green AR, Colado MI (2004) 3,4-Methylenedioxymethamphetamine increases interleukin-1β levels and activates microglia in rat brain: studies on the relationship with acute hyperthermia and 5-HT depletion. J Neurochem 89:1445–1453
Straiko MMW, Coolen LM, Zemlan FP, Gudelsky GA (2007) The effect of amphetamine analogs on cleaved microtubule-associated protein-tau formation in the rat brain. Neuroscience 144:223–231
Bendotti C, Baldessari S, Pende M, Tarizzo G, Miari A, Presti ML, Mennini T, Samanin R (1994) Does GFAP mRNA and mitochondrial benzodiazepine receptor binding detect serotonergic neuronal degeneration in rat? Brain Res Bull 34:389–394
Dugar A, Patanow C, O’Callaghan JP, Lakoski JM (1998) Immunohistochemical localization and quantification of glial fibrillary acidic protein and synaptosomal-associated protein (mol wt 25000) in the ageing hippocampus following administration of 5,7-dihydroxytryptamine. Neuroscience 85:123–133
Rowland NE, Kalehua AN, Li BH, Semple-Rowland SL, Streit WJ (1993) Loss of serotonin uptake sites and immunoreactivity in rat cortex after dexfenfluramine occur without parallel glial cell reaction. Brain Res 624:35–43
Williams MT, Brown CA, Skelton MR, Vinks AA, Vorhees CV (2004) Absorption and clearance of ±3,4-methylenedioxymethamphetamine from the plasma of neonatal rats. Neurotoxicol Teratol 26:849–856
Colado MI, O'Shea E, Granados R, Misra A, Murray TK, Green AR (1997) A study of the neurotoxic effect of MDMA (“ecstasy”) on 5-HT neurones in the brains of mothers and neonates following administration of the drug during pregnancy. Br J Pharmacol 121:827–833
Kelly PAT, Ritchie IM, Quate L, McBean DE, Olverman HJ (2002) Functional consequences of perinatal exposure to 3,4-methylenedioxymethamphetamine in rat brain. Br J Pharmacol 137:963–970
Campbell NG, Koprich JB, Kanaan NM, Lipton JW (2006) Mdma administration to pregnant Sprague–Dawley rats results in its passage to the fetal compartment. Neurotoxicol Teratol 28:459–465
Koprich JB, Chen E-Y, Kanaan NM, Campbell NG, Kordower JH, Lipton JW (2003) Prenatal 3,4-methylenedioxymethamphetamine (ecstasy) alters exploratory behavior, reduces monoamine metabolism, and increases forebrain tyrosine hydroxylase fiber density of juvenile rats. Neurotoxicol Teratol 25:509–517
Galineau L, Belzung C, Kodas E, Bodard S, Guilloteau D, Chalon S (2005) Prenatal 3,4-methylenedioxymethamphetamine (ecstasy) exposure induces long-term alterations in the dopaminergic and serotonergic functions in the rat. Brain Res Dev Brain Res 154:165–176
Koprich JB, Campbell NG, Lipton JW (2003) Neonatal 3,4-methylenedioxymethamphetamine (ecstasy) alters dopamine and serotonin neurochemistry and increases brain-derived neurotrophic factor in the forebrain and brainstem of the rat. Brain Res Dev Brain Res 147:177–182
Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV (2001) 3,4-Methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci 21:3228–3235
Crawford CA, Williams MT, Kohutek JL, Choi FY, Yoshida ST, McDougall SA, Vorhees CV (2006) Neonatal 3,4-methylenedioxymethamphetamine (MDMA) exposure alters neuronal protein kinase A activity, serotonin and dopamine content, and [35S]GTPgammaS binding in adult rats. Brain Res 1077:178–186
Williams MT, Schaefer TL, Ehrman LA, Able JA, Gudelsky GA, Sah R, Vorhees CV (2005) 3,4-Methylenedioxymethamphetamine administration on postnatal day 11 in rats increases pituitary–adrenal output and reduces striatal and hippocampal serotonin without altering SERT activity. Brain Res 1039:97–107
Meyer JS, Ali SF (2002) Serotonergic neurotoxicity of MDMA (ecstasy) in the developing rat brain. Ann NY Acad Sci 965:373–380
Dafters RI, Emily L (1998) Persistent loss of thermoregulation in the rat induced by 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) but not by fenfluramine. Psychopharmacology 138:207–212
Piper BJ, Meyer JS (2004) Memory deficit and reduced anxiety in young adult rats given repeated intermittent MDMA treatment during the periadolescent period. Pharmacol Biochem Behav 79:723–731
Marston HM, Reid ME, Lawrence JA, Olverman HJ, Butcher SP (1999) Behavioural analysis of the acute and chronic effects of MDMA treatment in the rat. Psychopharmacology 144:67–76
Sprague JE, Preston AS, Leifheit M, Woodside B (2003) Hippocampal serotonergic damage induced by MDMA (ecstasy): effects on spatial learning. Physiol Behav 79:281–287
Cohen AM, Skelton RM, Schaefer TL, Gudelsky GA, Vorhees C, Williams MT (2005) Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (ecstasy) in rats: interaction with exposure in adulthood. Synapse 57:148–159
Able JA, Gudelsky GA, Vorhees CV, Williams MT (2006) 3,4-Methylenedioxymethamphetamine in adult rats produces deficits in path integration and spatial reference memory. Biol Psychiatry 59:1219–1226
Slikker WJ, Ali SF, Scallet AC, Frith CH, Newport GD, Bailey JR (1988) Neurochemical and neurohistological alterations in the rat and monkey produced by orally administered methylenedioxymethamphetamine (MDMA). Toxicol Appl Pharmacol 96:448–457
Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW (1988) 3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. J Am Med Assoc 260:51–55
Ricaurte GA, Martello AL, Katz JL, Martello MB (1992) Lasting effects of (±)-3,4-methylenedioxymethamphetamine (MDMA) on central serotonergic neurons in nonhuman primates: neurochemical observations. J Pharmacol Exp Ther 261:616–622
Insel TR, Battaglia G, Johannessen JN, Marra S, De Souza EB (1989) 3,4-Methylenedioxymethamphetamine (“ecstasy”) selectively destroys brain serotonin terminals in rhesus monkeys. J Pharmacol Exp Ther 249:713–720
Hatzidimitriou G, McCann UD, Ricaurte GA (1999) Altered serotonin innervation patterns in the forebrain of monkeys treated with (±)3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery. J Neurosci 15:5096–5107
Ali SF, Newport GD, Scallet AC, Binienda Z, Ferguson SA, Bailey JR, Paule MG, Slikker JW (1993) Oral administration of 3,4-methylenedioxymethamphetamine (MDMA) produces selective serotonergic depletion in the nonhuman primate. Neurotoxicol Teratol 15:91–96
Ricaurte GA, DeLanney LE, Irwin I, Langston JW (1988) Toxic effects of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration. Brain Res 446:165–168
Ricaurte GA, Yuan J, McCann UD (2000) (±)3,4-Methylenedioxymethamphetamine (“ecstasy”)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 42:5–10
Mechan A, Yuan J, Hatzidimitriou G, Irvine RJ, McCann UD, Ricaurte GA (2005) Pharmacokinetic profile of single and repeated oral doses of MDMA in squirrel monkeys: relationship to lasting effects on brain serotonin neurons. Neuropsychopharmacology 31:339–350
Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza Ii R, Traynor JR, Woods JH (2008) A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience 151:533–543
Fornai F, Giorgi FS, Gesi M, Chen K, Alessri MG, Shih JC (2001) Biochemical effects of the monoamine neurotoxins DSP-4 and MDMA in specific brain regions of MAO-B-deficient mice. Synapse 39:213–221
Taraska T, Finnegan KT (1997) Nitric oxide and the neurotoxic effects of methamphetamine and 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther 280:941–947
Izco M, Marchant I, Escobedo I, Peraile I, Delgado M, Higuera-Matas A, Olias O, Ambrosio E, O’Shea E, Colado MI (2007) Mice with decreased cerebral dopamine function following a neurotoxic dose of MDMA (3,4-methylenedioxymethamphetamine, “ecstasy”) exhibit increased ethanol consumption and preference. J Pharmacol Exp Ther 322:1003–1012
Itzhak Y, Ali SF, Achat CN, Anderson KL (2003) Relevance of MDMA (“ecstasy”)-induced neurotoxicity to long-lasting psychomotor stimulation in mice. Psychopharmacology 166:241–248
Fornai F, Lenzi P, Frenzilli G, Gesi M, Ferrucci M, Lazzeri G, Biagioni F, Nigro M, Falleni A, Giusiani M, Pellegrini A, Blandini F, Ruggieri S, Paparelli A (2004) DNA damage and ubiquitinated neuronal inclusions in the substantia nigra and striatum of mice following MDMA (ecstasy). Psychopharmacology 173:353–363
Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM (2004) Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett 367:349–354
Fornai F, Gesi M, Lenzi P, Ferrucci M, Pellegrini A, Ruggieri S, Casini A, Paparelli A (2002) Striatal postsynaptic ultrastructural alterations following methylenedioxymethamphetamine administration. Ann N Y Acad Sci 965:381–398
Fornai F, Lazzeri G, Lenzi P, Gesi M, Ferrucci M, Soldani P, Pellegrini A, Capobianco L, Blasi A, Ruggieri S, Paparelli A (2003) Amphetamines induce ubiquitin-positive inclusions within striatal cells. Neurol Sci 24:182–183
Fornai F, Gesi M, Lenzi P, Ferrucci M, Lazzeri G, Pizzanelli C, Pellegrini A, Battaglia G, Ruggieri S, Paparelli A (2004) Effects of repeated low doses of MDMA on EEG activity and fluoro-jade B histochemistry. Ann NY Acad Sci 1025:181–188
Frenzilli G, Ferrucci M, Giorgi FS, Blandini F, Nigro M, Ruggieri S, Murri L, Paparelli A, Fornai F (2007) DNA fragmentation and oxidative stress in the hippocampal formation: a bridge between 3,4-methylenedioxymethamphetamine (ecstasy) intake and long-lasting behavioral alterations. Behav Pharmacol 18:471–481
Trigo J, Cabrero-Castel A, Berrendero F, Maldonado R, Robledo P (2008) MDMA modifies active avoidance learning and recall in mice. Psychopharmacology 197:391–400
Busceti CL, Biagioni F, Riozzi B, Battaglia G, Storto M, Cinque C, Molinaro G, Gradini R, Caricasole A, Canudas AM, Bruno V, Nicoletti F, Fornai F (2008) Enhanced tau phosphorylation in the hippocampus of mice treated with 3,4-methylenedioxymethamphetamine (“ecstasy”). J. Neurosci. 28:3234–3245
Renoir T, Païzanis E, Yacoubi ME, Saurini F, Hanoun N, Melfort M, Lesch KP, Hamon M, Lanfumey L (2008) Differential long-term effects of MDMA on the serotoninergic system and hippocampal cell proliferation in 5-HTt knock-out vs. wild-type mice. Int J Neuropsychopharmacol 11:1149–1162
Easton N, Marsden CA (2006) Ecstasy: are animal data consistent between species and can they translate to humans. J Psychopharmacol 20:194–210
Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS (2000) Striatal serotonin is depleted in brain of a human MDMA (ecstasy) user. Neurology 55:294–296
McCann U, Mertl M, Eligulashvili V, Ricaurte G (1999) Cognitive performance in 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) users: a controlled study. Psychopharmacology 143:417–425
McCann UD, Ridenour A, Shaham Y, Ricaurte GA (1994) Serotonin neurotoxicity after (+/−)3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”): a controlled study in humans. Neuropsychopharmacology 10:129–138
Bolla KI, McCann UD, Ricaurte GA (1998) Memory impairment in abstinent MDMA (“ecstasy”) users. Neurology 51:1532–1537
Cowan RL, Lyoo IK, Sung SM, Ahn KH, Kim MJ, Hwang J, Haga E, Vimal RLP, Lukas SE, Renshaw PF (2003) Reduced cortical gray matter density in human MDMA (ecstasy) users: a voxel-based morphometry study. Drug Alcohol Depend 72:225–235
McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA (1998) Positron emission tomographic evidence of toxic effect of MDMA (“ecstasy”) on brain serotonin neurones in human beings. Lancet 352:1433–1437
McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA (2005) Quantitative pet studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology 30:1741–1750
McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA (2008) Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (±)3,4-methylenedioxymethamphetamine (“ecstasy”) users: Relationship to cognitive performance. Psychopharmacology 3:439–450
Buchert R, Thomasius R, Nebeling B, Petersen K, Obrocki J, Jenicke L, Wilke F, Wartberg L, Zapletalova P, Clausen M (2003) Long-term effects of “ecstasy” use on serotonin transporters of the brain investigated by PET. J Nucl Med 44:375–384
Buchert R, Thomasius R, Wilke F, Petersen K, Nebeling B, Obrocki J, Schulze O, Schmidt U, Clausen M (2004) A voxel-based pet investigation of the long-term effects of “ecstasy” consumption on brain serotonin transporters. Am J Psychiatry 161:1181–1189
Thomasius R, Zapletalova P, Petersen K, Buchert R, Andresen B, Wartberg L, Nebeling B, Schmoldt A (2006) Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol 20:211–225
Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Gunning WB, den Heeten GJ, van den Brink W (2001) Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet 358:1864–1869
Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC (1999) Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (“ecstasy”) users. Br J Psychiatry 175:63–69
Reneman L, Endert E, Bruin KD, Lavalaye J, Feenstra M, Wolff FD, Booij J (2002) The acute and chronic effects of MDMA (“ecstasy”) on cortical 5-HT2a receptors in rat and human brain. Neuropsychopharmacology 26:387–396
Kish SJ (2002) How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy? Pharmacol Biochem Behav 71:845–855
Reneman L, Booij J, Habraken JB, De Bruin K, Hatzidimitriou G, Den Heeten GJ, Ricaurte GA (2002) Validity of [123I]beta-CIT SPECT in detecting MDMA-induced serotonergic neurotoxicity. Synapse 46:199–205
Veryheyden SL, Hadfield J, Calin T, Curran HV (2002) Sub-acute effects of MDMA (±3,4-methylenedioxymethamphetamine “ecstasy”) on mood: evidence of gender differences. Psychopharmacology 161:23–31
Rodgers J (2000) Cognitive performance amongst recreational users of “ecstasy”. Psychopharmacology 151:19–24
Creighton FJ, Black DL, Hyde CE (1991) “Ecstasy” psychosis and flashbacks. Br J Psychiatry 159:713–715
McGuire P (2000) Long-term psychiatric and cognitive effects of MDMA use. Toxicol Lett 112:153–156
Gouzoulis-Mayfrank E, Thimm B, Rezk M, Hensen G, Daumann J (2003) Memory impairment suggests hippocampal dysfunction in abstinent ecstasy users. Prog Neuropsychopharmacol Biol Psychiatry 27:819–827
Fox HC, McLean A, Turner JJ, Parrott AC, Rogers R, Sahakian BJ (2002) Neuropsychological evidence of a relatively selective profile of temporal dysfunction in drug-free MDMA (‘ecstasy’) polydrug users. Psychopharmacology 162:203–214
Moeller FG, Steinberg JL, Dougherty DM, Narayana PA, Kramer LA, Renshaw PF (2004) Functional MRI study of working memory in MDMA users. Psychopharmacology 177:185–194
Morgan M, McFie L, Fleetwood L, Robinson J (2002) Ecstasy (MDMA): are the psychological problems associated with its use reversed by prolonged abstinence. Psychopharmacology 159:294–303
Quednow BB, Jessen F, Kuhn K-U, Maier W, Daum I, Wagner M (2006) Memory deficits in abstinent MDMA (ecstasy) users: neuropsychological evidence of frontal dysfunction. J Psychopharmacol 20:373–384
Jacobsen LK, Mencl WE, Pugh KR, Skudlarski P, Krystal JH (2004) Preliminary evidence of hippocampal dysfunction in adolescent MDMA (“ecstasy”) users: possible relationship to neurotoxic effects. Psychopharmacology 173:383–390
Daumann J, Fischermann T, Heekeren K, Henke K, Thron A, Gouzoulis-Mayfrank E (2005) Memory-related hippocampal dysfunction in poly-drug ecstasy (3,4-methylenedioxymethamphetamine) users. Psychopharmacology 180:607–611
Schilt T, de Win MML, Koeter M, Jager G, Korf DJ, van den Brink W, Schmand B (2007) Cognition in novice ecstasy users with minimal exposure to other drugs: a prospective cohort study. Arch Gen Psychiatry 64:728–736
Morgan MJ, Impallomeni LC, Pirona A, Rogers RD (2006) Elevated impulsivity and impaired decision-making in abstinent ecstasy (MDMA) users compared to polydrug and drug-naïve controls. Neuropsychopharmacology 31:1562–1573
Butler GKL, Montgomery AMJ (2004) Impulsivity, risk taking and recreational “ecstasy” (MDMA) use. Drug Alcohol Depend 76:55–62
Daumann J, Fischermann T, Heekeren K, Thron A, Gouzoulis-Mayfrank E (2004) Neural mechanisms of working memory in ecstasy (MDMA) users who continue or discontinue ecstasy and amphetamine use: evidence from an 18-month longitudinal functional magnetic resonance imaging study. Biol Psychiatry 56:349–355
Reneman L, Booij J, Schmand B, Brink WVD, Gunning B (2000) Memory disturbances in “ecstasy” users are correlated with an altered brain serotonin neurotransmission. Psychopharmacology 148:322–324
Lesch KP, Gutknecht L (2005) Pharmacogenetics of the serotonin transporter. Prog Neuropsychopharmacol Biol Psychiatry 29:1062–1073
Roiser JP, Rogers RD, Cook LJ, Sahakian BJ (2006) The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology 188:213–227
Roiser JP, Cook LJ, Cooper JD, Rubinsztein DC, Sahakian BJ (2005) Association of a functional polymorphism in the serotonin transporter gene with abnormal emotional processing in ecstasy users. Am J Psychiatry 162:609–612
de Win MML, Schilt T, Reneman L, Vervaeke H, Jager G, Dijkink S, Booij J, van den Brink W (2006) Ecstasy use and self-reported depression, impulsivity, and sensation seeking: a prospective cohort study. J Psychopharmacol 20:226–235
Jager G, de Win M, Vervaeke H, Schilt T, Kahn R, van den Brink W, van Ree J, Ramsey N (2007) Incidental use of ecstasy: no evidence for harmful effects on cognitive brain function in a prospective fMRI study. Psychopharmacology 193:403–414
Dafters RI, Hoshi R, Talbot AC (2004) Contribution of cannabis and MDMA (“ecstasy”) to cognitive changes in long-term polydrug users. Psychopharmacology 173:405–410
Gamma A, Buck A, Berthold T, Vollenweider FX (2001) No difference in brain activation during cognitive performance between ecstasy (3,4-methylenedioxymethamphetamine) users and control subjects: a [H2(15)O]-positron emission tomography study. J Clin Psychopharmacol 21:66–71
O'Shea E, Escobedo I, Orio L, Sanchez V, Navarro M, Green AR, Colado MI (2005) Elevation of ambient room temperature has differential effects on MDMA-induced 5-HT and dopamine release in striatum and nucleus accumbens of rats. Neuropsychopharmacology 30:1312–1323
Farfel GM, Seiden LS (1995) Role of hypothermia in the mechanism of protection against serotonergic toxicity. Experiments using 3,4-methylenedioxymethamphetamine, dizocilpine, CGS 19755 and NBQX. J Pharmacol Exp Ther 272:860–867
Colado MI, Granados R, O’Shea E, Esteban B, Green AR (1998) Role of hyperthermia in the protective action of chlomethiazole against MDMA (“ecstasy”)-induced neurodegeneration, comparison with the novel NMDA channel blocker AR-R15896AR. Br J Pharmacol 124:479–484
Farfel GM, Vosmer GL, Seiden LS (1992) The antagonist MK-801 protects against serotonin depletions induced by methamphetamine 3,4-methylenedioxymethamphetamine and p-chloroamphetamine. Brain Res 595:121–127
Johnson M, Bush LG, Hanson GR, Gibb JW (1993) Effects of ritanserin on the 3,4-methylenedioxymethamphetamine-induced decrease in striatal serotonin concentration and on the increase in striatal neurotensin and dynorphin A concentrations. Biochem Pharmacol 46:770–772
Stone DM, Johnson M, Hanson GR, Gibb JW (1988) Role of endogenous dopamine in the central serotonergic deficits induced by 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther 247:79–87
Colado MI, O'Shea E, Granados R, Murray TK, Green AR (1997) In vivo evidence for free radical involvement in the degeneration of rat brain 5-HT following administration of MDMA (“ecstasy”) and p-chloroamphetamine but not the degeneration following fenfluramine. Br J Pharmacol 121:889–900
Colado MI, O'Shea E, Esteban B, Granados R, Green AR (1999) In vivo evidence against clomethiazole being neuroprotective against MDMA (“ecstasy”)-induced degeneration of rat brain 5-HT nerve terminals by a free radical scavenging mechanism. Neuropharmacology 38:307–314
Gordon CJ, Fogelson L (1994) Metabolic and thermoregulatory responses of the rat maintained in acrylic or wire-screen cages: implications for pharmacological studies. Physiol Behav 56:73–79
Nagatsu T (2004) Progress in monoamine oxidase (MAO) research in relation to genetic engineering. Neurotoxicology 25:11–20
Alves E, Summavielle T, Alves CJ, Gomes-da-Silva J, Barata JC, Fernandes E, de Lourdes Bastos M, Tavares MA, Carvalho F (2007) Monoamine oxidase-B mediates ecstasy-induced neurotoxic effects to adolescent rat brain mitochondria. J. Neurosci. 27:10203–10210
Sprague JE, Everman SL, Nichols DE (1998) An integrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine. Neurotoxicology 19:427–441
Sprague JE, Nichols DE (1995) The monoamine oxidase-B inhibitor l-deprenyl protects against 3,4-methylenedioxymethamphetamine-induced lipid peroxidation and long-term serotonergic deficits. J Pharmacol Exp Ther 273:667–673
Marchitti SA, Deitrich RA, Vasiliou V (2007) Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev 59:125–150
Falk EM, Cook VJ, Nichols DE, Sprague JE (2002) An antisense oligonucleotide targeted at MAO-B attenuates rat striatal serotonergic neurotoxicity induced by MDMA. Pharmacol Biochem Behav 72:617–622
Suliman HB, Carraway MS, Velsor LW, Day BJ, Ghio AJ, Piantadosi CA (2002) Rapid mtDNA deletion by oxidants in rat liver mitochondria after hemin exposure. Free Radic Biol Med 32:246–256
Suliman HB, Carraway MS, Piantadosi CA (2003) Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med 167:570–579
Alves E, Summavielle T, Alves CJ, Gomes-da-Silva J, Barata JC, Fernandes E, Bastos ML, Tavares MA, Carvalho F (2009) Ecstasy-induced oxidative stress to adolescent rat brain mitochondria in vivo: Influence of monoamine oxidase type A. Addict Biol. 14:185–193
Fowler CJ, Tipton KF (1982) Deamination of 5-hydroxytryptamine by both forms of monoamine oxidase in the rat brain. J Neurochem 38:733–736
Amato JL, Bankson MG, Yamamoto BK (2006) Prior exposure to chronic stress and MDMA potentiates mesoaccumbens dopamine release mediated by the 5-HT1b receptor. Neuropsychopharmacology 32:946–954
Schmidt CJ, Black CK, Taylor VL (1990) Antagonism of the neurotoxicity due to a single administration of methylenedioxymethamphetamine. Eur J Pharmacol 181:59–70
Shankaran M, Yamamoto BK, Gudelsky GA (1999) Mazindol attenuates the 3,4-methylenedioxymethamphetamine-induced formation of hydroxyl radicals and long-term depletion of serotonin in the striatum. J Neurochem 72:2516–2522
Kanthasamy A, Sprague JE, Shotwell JR, Nichols DE (2002) Unilateral infusion of a dopamine transporter antisense into the substantia nigra protects against MDMA-induced serotonergic deficits in the ipsilateral striatum. Neuroscience 114:917–924
Stone DM, Johnson M, Hanson GR, Gibb JW (1989) Acute inactivation of tryptophan hydroxylase by amphetamine analogs involves the oxidation of sulfhydryl sites. Eur J Pharmacol 7:93–97
Remião F, Rettori D, Han D, Carvalho F, Bastos ML, Cadenas E (2004) Leucoisoprenochrome-o-semiquinone formation in freshly isolated adult rat cardiomyocytes. Chem Res Toxicol 17:1584–1590
Costa VM, Silva R, Ferreira LM, Branco PS, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F (2007) Oxidation process of adrenaline in freshly isolated rat cardiomyocytes: formation of adrenochrome, quinoproteins and GSH-adduct. Chem Res Toxicol 20:1183–1191
Bindoli A, Rigobello MP, Deeble DJ (1992) Biochemical and toxicological properties of the oxidation products of catecholamines. Free Radic Biol Med 13:391–405
Remião F, Milhazes N, Borges F, Carvalho F, Bastos ML, Lemos-Amado F, Domingues P, Ferrer-Correia A (2003) Synthesis and analysis of aminochromes by HPLC-photodiode array. Adrenochrome evaluation in rat blood. Biomed Chromatogr 17:6–13
Spencer JPE, Jenner P, Daniel SE, Lees AJ, Marsden DC, Halliwell B (1998) Conjugates of catecholamines with cysteine and GSH in Parkinson’s disease: possible mechanism of formation involving reactive oxygen species. J Neurochem 71:2112–2122
Spencer JPE, Whiteman M, Jenner P, Halliwel B (2002) Cysteinyl-conjugates of catecholamines induce cell damage, extensive DNA base modification and increases in caspase-3 activity in neurons. J Neurochem 81:122–129
Yamamoto BK, Nash JF, Gudelsky GA (1995) Modulation of methylenedioxymethamphetamine-induced striatal dopamine release by the interaction between serotonin and gamma-aminobutyric acid in the substantia nigra. J Pharmacol Exp Ther 273:1063–1070
Bankson MG, Yamamoto BK (2004) Serotonin-GABA interactions modulate MDMA-induced mesolimbic dopamine release. J Neurochem 91:852–859
Breier JM, Bankson MG, Yamamoto BK (2006) l-Tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. J Neurosci 26:290–299
Darvesh AS, Yamamoto BK, Gudelsky GA (2005) Evidence for the involvement of nitric oxide in 3,4-methylenedioxymethamphetamine-induced serotonin depletion in the rat brain. J Pharmacol Exp Ther 312:694–701
Goñi-Allo B, Puerta E, Mathúna BO, Hervias I, Lasheras B, de la Torre R, Aguirre N (2008) On the role of tyrosine and peripheral metabolism in 3,4-methylenedioxymethamphetamine-induced serotonin neurotoxicity in rats. Neuropharmacology 54:885–900
Shankaran M, Gudelsky GA (1998) Effect of 3,4-methylenedioxymethamphetamine (MDMA) on hippocampal dopamine and serotonin. Pharmacol Biochem Behav 61:361–366
Yuan J, Cord BJ, McCann UD, Callahan BT, Ricaurte GA (2002) Effect of depleting vesicular and cytoplasmic dopamine on methylenedioxymethamphetamine neurotoxicity. J Neurochem 80:960–969
Wrona MZ, Yang Z, McAdams M, O’Connor-Coates S, Dryhurst G (1995) Hydroxyl radical-mediated oxidation of serotonin: potential insights into the neurotoxicity of methamphetamine. Journal of Neurochemistry 64:1390–1400
Wrona MZ, Dryhurst G (1998) Oxidation of serotonin by superoxide radical: implications to neurodegenerative brain disorders. Chem Res Toxicol 11:639–650
Jiang XR, Wrona MZ, Dryhurst G (1999) Tryptamine-4,5-dione, a putative endotoxic metabolite of the superoxide-mediated oxidation of serotonin, is a mitochondrial toxin: possible implications in neurodegenerative brain disorders. Chem Res Toxicol 12:429–436
Rothman RB, Baumann MH (2003) Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479:23–40
Jiang XR, Dryhurst G (2002) Inhibition of the α-ketoglutarate dehydrogenase and pyruvate dehydrogenase complexes by a putative aberrant metabolite of serotonin, tryptamine-4,5-dione. Chem Res Toxicol 15:1242–1247
Wrona MZ, Dryhurst G (2001) A putative metabolite of serotonin, tryptamine-4,5-dione, is an irreversible inhibitor of tryptophan hydroxylase: possible relevance to the serotonergic neurotoxicity of methamphetamine. Chem Res Toxicol 14:1184–1192
Johnson M, Mitros K, Stone DM, Zobrist R, Hanson GR, Gibb JW (1992) Effect of flunarizine and nimodipine on the decrease in tryptophan hydroxylase activity induced by methamphetamine and 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther 261:586–591
Bonkale WL, Austin MC (2008) 3,4-Methylenedioxymethamphetamine induces differential regulation of tryptophan hydroxylase 2 protein and mRNA levels in the rat dorsal raphe nucleus. Neuroscience 155:270–276
Elayan I, Gibb JW, Hanson GR, Lim HK, Foltz RL, Johnson M (1993) Short-term effects of 2,4,5-trihydroxyamphetamine, 2,4,5-trihydroxymethamphetamine and 3,4-dihydroxymethamphetamine on central tryptophan hydroxylase activity. J Pharmacol Exp Ther 265:813–818
Kuhn DM, Arthur RA Jr (1997) Molecular mechanism of the inactivation of tryptophan hydroxylase by nitric oxide: attack on critical sulfhydryls that spare the enzyme iron center. J Neurosci 17:7245–7251
Hemeryck A, Belpaire FM (2002) Selective serotonin reuptake inhibitors and cytochrome p-450 mediated drug–drug interactions: an update. Curr Drug Metab 3:13–37
Upreti VV, Eddington ND (2008) Fluoxetine pretreatment effects pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) in rat. J Pharm Sci 97:1593–1605
Yamamoto Y, Tasaki T, Nakamura A, Iwata H, Kazusaka A, Gonzalez FJ, Fujita S (1998) Molecular basis of the Dark Agouti rat drug oxidation polymorphism: importance of CYP2D1 and CYP2D2. Pharmacogenetics 8:73–82
Kawase A, Fujii A, Negoro M, Akai R, Ishikubo M, Komura H, Iwaki M (2008) Differences in cytochrome p450 and nuclear receptor mRNA levels in liver and small intestines between SD and DA rats. Drug Metab Pharmacokinet 23:196–206
Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD (1998) Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem 273:2458–2466
Kramer HK, Poblete JC, Azmitia EC (1995) 3,4-Methylenedioxymethamphetamine (“ecstasy”) promotes the translocation of protein kinase C (PKC): requirement of viable serotonin nerve terminals. Brain Res 680:1–8
Kramer HK, Poblete JC, Azmitia EC (1997) Activation of protein kinase C (PKC) by 3,4-methylenedioxymethamphetamine (MDMA) occurs through the stimulation of serotonin receptors and transporter. Neuropsychopharmacology 17:117–129
Kramer HK, Poblete JC, Azmitia EC (1998) Characterization of the translocation of protein kinase C (PKC) by 3,4-methylenedioxymethamphetamine (MDMA/ecstasy) in synaptosomes: evidence for a presynaptic localization involving the serotonin transporter (SERT). Neuropsychopharmacology 19:265–277
Yi J-H, Hazell AS (2006) Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int 48:394–403
Nash JF, Yamamoto BK (1992) Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3, 4-methylenedioxymethamphetamine. Brain Res 581:237–243
White SR, Duffy P, Kalivas PW (1994) Methylenedioxymethamphetamine depresses glutamate-evoked neuronal firing and increases extracellular levels of dopamine and serotonin in the nucleus accumbens in vivo. Neuroscience 62:41–50
Obradovic T, Imel KM, White SR (1996) Methylenedioxymethamphetamine-induced inhibition of neuronal firing in the nucleus accumbens is mediated by both serotonin and dopamine. Neuroscience 74:469–481
Chipana C, Torres I, Camarasa J, Pubill D, Escubedo E (2008) Memantine protects against amphetamine derivatives-induced neurotoxic damage in rodents. Neuropharmacology 54:1254–1263
Kindlundh-Hogberg A, Blomqvist A, Malki R, Schioth H (2008) Extensive neuroadaptive changes in cortical gene-transcript expressions of the glutamate system in response to repeated intermittent MDMA administration in adolescent rats. BMC Neurosci 9:39
Pannu R, Singh I (2006) Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int 49:170–182
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 87:1620–1624
Zheng Y, Laverty R (1998) Role of brain nitric oxide in (±)3,4-methylenedioxymethamphetamine (MDMA)-induced neurotoxicity in rats. Brain Res 795:257–263
Globus M, Busto R, Lin B, Schnippering H, Ginsberg M (1995) Detection of free radical activity during transient global ischemia and recirculation: effects of intraischemic brain temperature modulation. J Neurochem 65:1250–1256
Kil H, Zhang J, Piantadosi C (1996) Brain temperature alters hydroxyl radical production during cerebral ischemia/reperfusion in rats. J Cereb Blood Flow Metab 16:100–106
Itzhak Y, Anderson KL, Ali SF (2004) Differential response of nNOS knockout mice to MDMA (“ecstasy”)-and methamphetamine-induced psychomotor sensitization and neurotoxicity. Ann N Y Acad Sci 1025:119–128
Itzhak Y, Gandia C, Huang PL, Ali SF (1998) Resistance of neuronal nitric oxide synthase-deficient mice to methamphetamineinduced dopaminergic neurotoxicity. J Pharmacol Exp Ther 284:1040–1047
Itzhak Y, Ali SF (2006) Role of nitrergic system in behavioral and neurotoxic effects of amphetamine analogs. Pharmacol Ther 109:246–262
Willins D, Deutch A, Roth B (1997) Serotonin 5-HT2a receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 27:79–82
Xu T, Pandey SC (2000) Cellular localization of serotonin2A (5HT2a) receptors in the rat brain. Brain Res Bull 51:499–505
O'Shea E, Sanchez V, Orio L, Escobedo I, Green AR, Colado MI (2005) 3,4-Methylenedioxymethamphetamine increases pro-interleukin-1β production and caspase-1 protease activity in frontal cortex, but not in hypothalamus, of dark agouti rats: role of interleukin-1β in neurotoxicity. Neuroscience 135:1095–1105
Arai K, Ikegaya Y, Nakatani Y, Kudo I, Nishiyama N, Matsuki N (2001) Phospholipase A2 mediates ischemic injury in the hippocampus: a regional difference of neuronal vulnerability. Eur J Neurosci 13:2319–2323
Azmitia E (2001) Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 56:413–424
Meltzer HY (1999) The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 21:106–115
Klisch J, Bode-Greuel KM, Horvath E, Klisch C, Els T (2003) Additive neuroprotective effect of ketanserin and ipsapirone on the hippocampal damage after transient forebrain ischemia in the Mongolian gerbil. Neurosci Lett 342:25–28
Abekawa T, Ito K, Nakagawa S, Nakato Y, Koyama T (2008) Olanzapine and risperidone block a high dose of methamphetamine-induced schizophrenia-like behavioral abnormalities and accompanied apoptosis in the medial prefrontal cortex. Schizophr Res 101:84–94
Esteban B, O'Shea E, Camarero J, Sanchez V, Green AR, Colado MI (2001) 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacology 154:251–260
Paris JM, Cunningham K (1992) Lack of serotonin neurotoxicity after intraraphe microinjection of (+)-3,4-methylenedioxymethamphetamine (MDMA). Brain Res Bull 28:115–119
Monks TJ, Jones DC, Fengju B, Lau SS (2004) The role of metabolism in 3,4-methylenodioxyamphetamine 3,4-methylenodioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit 26:132–136
Erives GV, Lau SS, Monks TJ (2008) Accumulation of neurotoxic thioether metabolites of 3,4-({±})-methylenedioxymethamphetamine in rat brain. J Pharmacol Exp Ther 324:284–291
Miller RT, Lau SS, Monks TJ (1996) Effects of intracerebroventricular administration of 5-(glutathion-S-yl)-α-methyldopamine on brain dopamine, serotonin, and norepinephrine concentrations in male Sprague–Dawley rats. Chem Res Toxicol 9:457–465
McCann UD, Ricaurte GA (1991) Major metabolites of (±)3,4-methylenedioxyamphetamine (MDA) do not mediate its toxic effects on brain serotonin neurons. Brain Res 545:279–282
Escobedo I, O’Shea E, Orio L, Sanchez V, Segura M, de la Torre R, Farre M, Green AR, Colado MI (2005) A comparative study on the acute and long-term effects of MDMA and 3,4-dihydroxymethamphetamine (HHMA) on brain monoamine levels after i.p. or striatal administration in mice. Br J Pharmacol 144:231–241
Yeh SY, Hsu F-L (1991) The neurochemical and stimulatory effects of putative metabolites of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine in rats. Pharmacol Biochem Behav 39:787–790
Miller RT, Lau SS, Monks TJ (1997) 2,5-Bis-(glutathion-S-yl)-α-methyldopamine, a putative metabolite of (±)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations. Eur J Pharmacol 323:173–180
Bai F, Lau SS, Monks TJ (1999) Glutathione and N-acetylcysteine conjugates of α-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol 12:1150–1157
Jones DC, Lau SS, Monks TJ (2004) Thioether metabolites of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine inhibit human serotonin transporter (hSERT) function and simultaneously stimulate dopamine uptake into hSERT-expressing SK-N-MC cells. J Pharmacol Exp Ther 311:298–306
Macedo C, Branco P, Ferreira L, Lobo A, Capela JP, Fernandes E, Bastos MDL, Carvalho F (2007) Synthesis and cyclic voltammetry studies of 3,4-methylenedioxymethamphetamine (MDMA) main human metabolites. J Health Sci 53:31–42
Kleiner HE, Rivera MI, Pumford NR, Monks TJ, Lau SS (1998) Immunochemical detection of quinol-thioether derived covalent protein adducts. Chem Res Toxicol 11:1283–1290
Miyazaki I, Asanuma M, Diaz-Corrales FJ, Fukuda M, Kitaichi K, Miyoshi K, Ogawa N (2006) Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinone formation-related molecules. FASEB J 20:571–573
Felim A, Urios A, Neudorffer A, Herrera G, Blanco M, Largeron M (2007) Bacterial plate assays and electrochemical methods: an efficient tandem for evaluating the ability of catechol-thioether metabolites of MDMA (“ecstasy”) to induce toxic effects through redox-cycling. Chem Res Toxicol 20:685–693
Remião F, Carvalho M, Carmo H, Carvalho F, Bastos ML (2002) Cu2+-induced isoproterenol oxidation into isoprenochrome in adult rat calcium-tolerant cardiomyocytes. Chem Res Toxicol 15:861–869
Patel NJ, Fullone JS, Anders MW (1993) Brain uptake of S-(1,2-dichlorovinyl) glutathione and S-(1,2-dichlorovinyl)-l-cysteine, the glutathione and cysteine S-conjugates of the neurotoxin dichloroacetylene. Brain Res Mol Brain Res 17:53–58
del Amo EM, Urtti A, Yliperttula M (2008) Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci 35:161–174
Li S, Whorton AR (2005) Identification of stereoselective transporters for S-nitroso-l-cysteine: role of LAT1 and LAT2 in biological activity of S-nitrosothiols. J Biol Chem 280:20102–20110
Shen XM, Dryhurst G (1996) Further insights into the influence of l-cysteine on the oxidation chemistry of dopamine: reaction pathways of potential relevance to Parkinson’s disease. Chem Res Toxicol 9:751–763
Wan F-J, Tung C-S, Shiah IS, Lin H-C (2006) Effects of [alpha]-phenyl-N-tert-butyl nitrone and N-acetylcysteine on hydroxyl radical formation and dopamine depletion in the rat striatum produced by d-amphetamine. Eur Neuropsychopharmacol 16:147–153
Hart AM, Terenghi G, Kellerth JO, Wiberg M (2004) Sensory neuroprotection, mitochondrial preservation, and therapeutic potential of N-acetyl-cysteine after nerve injury. Neuroscience 125:91–101
Hussain S, Slikker Jr W, Ali SF (1996) Role of metallothionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection. Neurochem Int 29:145–152
Sekhon B, Sekhon C, Khan M, Patel SJ, Singh I, Singh AK (2003) N-acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res 971:1–8
Gudelsky G (1996) Effect of ascorbate and cysteine on the 3,4-methylenedioxymethamphetamine-induced depletion of brain serotonin. J Neural Transm 103:1397–1404
Shankaran M, Yamamoto BK, Gudelsky GA (2001) Ascorbic acid prevents 3,4-methylenedioxymethamphetamine (MDMA)-induced hydroxyl radical formation and the behavioral and neurochemical consequences of the depletion of brain 5-HT. Synapse 40:55–64
Alves E, Binienda Z, Carvalho F, Alves CJ, Fernandes E, de Lourdes Bastos M, Tavares MA, Summavielle T (2009) Acetyl-l-carnitine provides effective in vivo neuroprotection over 3,4-methylenedioximethamphetamine-induced mitochondrial neurotoxicity in the adolescent rat brain. Neuroscience 158:514–523
Slot AJ, Wise DD, Deeley RG, Monks TJ, Cole SPC (2008) Modulation of human multidrug resistance protein (MRP) 1 (ABCC1) and MRP2 (ABCC2) transport activities by endogenous and exogenous glutathione-conjugated catechol metabolites. Drug Metab Dispos 36:552–560
Ballatori N, Krance SM, Marchan R, Hammond CL (2009) Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Aspects Med. doi:10.1016/j.mam.2008.08.004
Costa VM, Ferreira LM, Branco PS, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F (2009) Cross-functioning between the extraneuronal monoamine transporter and multidrug resistance protein 1 in the uptake of adrenaline and export of 5-(glutathion-S-yl)adrenaline in rat cardiomyocytes. Chem Res Toxicol 22:129–135
Acknowledgments
J.P.C. acknowledges “Fundação para a Ciência e a Tecnologia” (FCT) Portugal for his post-doc grant (ref. SFRH/BPD/30776/2006).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Capela, J.P., Carmo, H., Remião, F. et al. Molecular and Cellular Mechanisms of Ecstasy-Induced Neurotoxicity: An Overview. Mol Neurobiol 39, 210–271 (2009). https://doi.org/10.1007/s12035-009-8064-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-009-8064-1