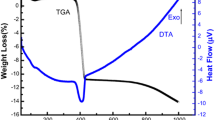
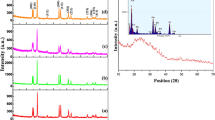
Abstract
Nanoparticles of sodium lanthanum (III) fluoride-doped and co-doped with Eu3+/Tb3+ were prepared by the hydrothermal method using citric acid as structure-directing agent. Structural aspects and optical properties of synthesized nanoparticles were studied by powder X-ray diffraction (XRPD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), selected area electron diffraction (SAED), energy-dispersive X-ray spectra (EDS), particle size by dynamic light scattering (DLS), Fourier transform infrared (FTIR) spectrum and photoluminescence (PL) techniques. Nanoparticles consist of well-crystallized hexagonal phase and the average crystallite size for undoped and doped-NaLaF4 nanoparticles are in the range of 20–22 nm. TEM images show that nanoparticles have cylindrical shape and crystalline nature of nanoparticles was confirmed by SAED patterns. Down- conversion (DC) luminescent properties of doped NaLaF4 were also investigated and impact of co-doping has been explored.













Similar content being viewed by others
References
Huang X, Han S, Huang W and Liu X 2013 Chem. Soc. Rev. 42 173
Bunzli J C G, Comby S, Chauvin A S and Vandevyver C D B 2007 J. Rare Earths 25 257
Mahalingam V, Mangiarini F, Vetrone F, Venkatramu V, Bettinelli M, Speghini A et al 2008, J. Phys. Chem. 112 17745
Kumar G A, Chen C W, Ballato J and Riman R E 2007 J. Mater. Chem. 19 1523
Liu H, Wang H, Zang X and Chen D 2009 J. Mater. Chem. 19 489
Evanics F, Diamente P R, van Veggel F C, Stanisz G J and Prosser R S 2006 J. Mater. Chem. 8 2499
Kumar R, Nyk M, Ohulchanskyy T Y, Flask C A and Prasad P N 2009 Adv. Funct. Mater. 19 853
Auzel F 2004 Chem. Rev. 104 139
Downing E, Hesselink L, Ralston J and Macfarlane R 1996 Science 273 1185
Jacinto C, Vermelho M, Gouveia E, de Araujo M, Udo P, Astrath N et al 2007, Appl. Phys. Lett. 91 071102
van der Ende B M, Aarts L and Meijerink A 2009 Phys. Chem. Chem. Phys. 11 11081
Nyk M, Kumar R, Ohulchanskyy T Y, Bergy E J and Prasad P N 2008 Nano Lett. 8 3834
Qin X, Yokomori T and Ju Y G 2007 Appl. Phys. Lett. 90 073104
Shan J S and Ju Y G 2007 Appl. Phys. Lett. 91 123103
Kramer K W, Biner D, Frei G, Gudel H U, Hehlen M P and Luthi S R 2004 Chem. Mater. 16 1244
Li C, Yang J, Yang P, Lian H and Lin J 2008 Chem. Mater. 20 4317
Xia Z G and Du P 2010 J. Mater. Res. 25 2035
Zeng S J, Ren G Z, Xu C F and Yang Q B 2011 Cryst. Eng. Comm. 13 4276
Ghosh P and Patra A 2008 J. Phys. Chem. 112 19283
Ghosh P and Patra A 2008 J. Phys. Chem. 112 3223
Ghosh P, Kar A and Patra A K 2010 J. Phys. Chem. C 114 715
Chen G Y, Ohulchanskyy T Y, Kachynski A, Agren H and Prasad P N 2011 AC Nano 5 4981
Teng X, Zhu Y, Wei W, Wang S, Huang J, Naccache R et al 2012, J. Am. Chem. Soc. 134 8340
Shang M, Li G, Kang X, Yang D, Geng D, Peng C et al 2012, Dalton Trans. 41 5571
Shang M M, Geng D L, Kang X J, Yang D M, Zhang Y and Lin J 2012 Inorg. Chem. 51 11106
He M, Huang P, Zhang C L, Hu H Y, Bao C C, Gao G et al 2011, Adv. Funct. Mater. 21 4470
Zhou J, Zhu X J, Chen M, Sun Y and Li F Y 2012 Biomaterials 33 6201
Li F, Li C, Liu X, Chen Y, Bai T, Wang L et al 2012, Chem. Eur. J. 18 11641
Dawer A L, Shishodia P K, Chouhan J, Kumar G and Mathur A 1990 Mater. Sci. Lett. 9 547
Tian Z R, Voigt J A, Liu J, Mckenzie B, Mcdermott M J, Rodriguez M A et al 2003, Nat. Mater. 2 821
Sun Y J, Chen Y, Tian L J, Yu Y, Kong X G, Zhao J W et al 2007, Nanotechnology 18 275609
Whitesides G M and Grzybowski B 2002 Science 295 2418
Wang Z L, Hao J H and Chan H L W 2010 Cryst. Eng. Comm. 12 1373
Zhao J W, Sun Y J, Kong X G, Tian L J, Wang Y, Tu L P et al 2008, J. Phys. Chem. 112 15666
Wang F, Han Y, Lim C, Lu Y, Wang J, Xu J et al 2010, Nature 463 1061
Eliseevaa S V and Bunzli J C G 2010 Chem. Soc. Rev. 39 189
Bunzli J C G and Piguet C 2005 Chem. Soc. Rev. 34 1048
Guangshun Y, Lee W B and Chow G M 2007 J. Nanosci. Nanotechnol. 7 2790
Binnemans K 2009 Chem. Soc. Rev. 109 4283
Gaft M H, Reisfeld R and Panczer G 2005 Luminescence spectroscopy of minerals and materials, 2nd edn (Berlin, Heidelberg: Springer)
Kirby A F, Foster D and Richardson F S 1983 Chem. Phys. Lett. 95 507
Kirby A F and Richardson F S 1983 J. Phys. Chem. 87 2544
Ghosh P and Patra A 2008 J. Phys. Chem. C 112 19283; 112 3223
Acknowledgements
We would like to acknowledge Indian Institute of Technology Roorkee and Indian Institute of Technology Guwahati for their technical support. We also thank School of Physics, Shri Mata Vaishno Devi University (SMVDU) for photoluminescence studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LADOL, J., KHAJURIA, H., KHAJURIA, S. et al. Hydrothermal synthesis, characterization and luminescent properties of lanthanide-doped NaLaF4 nanoparticles. Bull Mater Sci 39, 943–952 (2016). https://doi.org/10.1007/s12034-016-1225-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-016-1225-8