Abstract
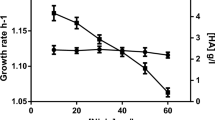
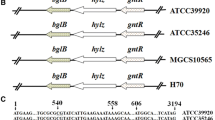
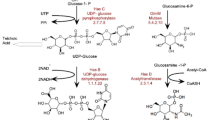
Despite the significant potential of Streptococcus zooepidemicus for hyaluronic acid (HA) production with high molecular weight (MW), the HA degrading properties of hyaluronidase prevents the bacteria to achieve enhanced HA yield with high MW. In the present study, we aim to knockout the hyaluronidase enzyme and assess its effects on the yield and MW of the produced HA. The kanamycin resistance gene between the left and right arms of hyaluronidase gene was inserted into pUC18 plasmid to construct pUC18-L-kanar-R as a recombinant suicide plasmid. The construct was then transferred into S. zooepidemicus to induce the homologous recombination between the hyaluronidase gene and the kanamycin resistance gene. Gene deletion was confirmed by PCR and enzyme assay. The product was cultured on selectable medium in which the MW of HA was increased from 1.5 to 3.8 MDa. The yield of HA production using the mutant strain was higher in all different concentrations of glucose from 40 to 120 g/l. Moreover, glucose increase results in higher HA production within both wild-type and recombinant strains. However, the growth rate of HA concentration (the slope of the plot), as a consequence of increased glucose concentration, is always higher for the recombinant strain. Unlike the wild-type strain, there was no sharp HA production drop approaching the 6 g/l HA concentration. In conclusion, hyaluronidase activity and HA concentration and MW exhibited a mutual control on each other. Based on our results, deletion of the hyaluronidase gene positively affects the yield and MW of HA.



Similar content being viewed by others
References
Meyer, K., & Palmer, J. W. (1934). The polysaccharide of the vitreous humor. Journal of Biological Chemistry, 107(3), 629–634.
Balazs, E. A., Leshchiner, E., Larsen, N. E., & Band, P. (1993). Application of hyaluronan and its derivatives. Biotechnological polymers. Technomic, Lancaster, 41–65.
Burdick, J. A., & Prestwich, G. D. (2011). Hyaluronic acid hydrogels for biomedical applications. Advanced Materials, 23(12), 41–56. doi:10.1002/adma.201003963.
Choh, S. Y., Cross, D., & Wang, C. (2011). Facile synthesis and characterization of disulfide-cross-linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules, 12, 1126–1136. doi:10.1021/bm101451k.
Kablik, J., Monheit, G. D., Yu, L., Chang, G., & Gershkovich, J. (2009). Comparative physical properties of hyaluronic acid dermal fillers. Dermatologic Surgery, 35(SUPPL. 1), 302–312. doi:10.1111/j.1524-4725.2008.01046.x.
Taylor, P., Hahn, S. K., Jelacic, S., Maier, R. V, Patrick, S., & Hoffman, A. S. (2012). Anti-inflammatory drug delivery from hyaluronic acid hydrogels. Journal of Biomaterials Science, 15, 37–41.
Mero, A., & Campisi, M. (2014). Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers, 6(1), 346–369. doi:10.3390/polym6020346.
Chong, B. F., Blank, L. M., Mclaughlin, R., & Nielsen, L. K. (2005). Microbial hyaluronic acid production. Applied Microbiology and Biotechnology, 66(4), 341–351. doi:10.1007/s00253-004-1774-4.
D’Ayala, G. G., Malinconico, M., & Laurienzo, P. (2008). Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules, 13(9), 2069–2106. doi:10.3390/molecules13092069.
Kim, J., Park, Y., Tae, G., Kyu, B. L., Chang, M. H., Soon, J. H., et al. (2009). Characterization of low-molecular-weight hyaluronic acid-based hydrogel and differential stem cell responses in the hydrogel microenvironments. Journal of Biomedical Materials Research—Part A, 88(4), 967–975. doi:10.1002/jbm.a.31947.
Kim, J., Kim, I. S., Cho, T. H., Lee, K. B., Hwang, S. J., Tae, G., et al. (2007). Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials, 28(10), 1830–1837. doi:10.1016/j.biomaterials.2006.11.050.
Marcellin, E., Steen, J. A., & Nielsen, L. K. (2014). Insight into hyaluronic acid molecular weight control. Applied Microbiology and Biotechnology, 98(16), 6947–6956. doi:10.1007/s00253-014-5853-x.
Lai, Z. W., Rahim, R. A., Ariff, A. B., & Mohamad, R. (2012). Biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus using oxygen vector and optimum impeller tip speed. Journal of Bioscience and Bioengineering, 114(3), 286–291. doi:10.1016/j.jbiosc.2012.04.011.
Choi, S., Choi, W., Kim, S., Lee, S.-Y., Noh, I., & Kim, C.-W. (2014). Purification and biocompatibility of fermented hyaluronic acid for its applications to biomaterials. Biomaterials Research, 18(1), 6. doi:10.1186/2055-7124-18-6.
O’Regan, M., Martini, I., Crescenzi, F., De Luca, C., & Lansing, M. (1994). Molecular mechanisms and genetics of hyaluronan biosynthesis. International Journal of Biological Macromolecules, 16(6), 283–286. doi:10.1016/0141-8130(94)90056-6.
Wei, Z., Fu, Q., Chen, Y., Cong, P., Xiao, S., Mo, D., et al. (2012). The capsule of Streptococcus equi ssp. zooepidemicus is a target for attenuation in vaccine development. Vaccine, 30(31), 4670–4675. doi:10.1016/j.vaccine.2012.04.092.
Schiraldi, C., Gatta, A. La, & De Rosa, M. (2010). Biotechnological production and application of hyaluronan. Biopolymers. doi:10.5772/10271.
Mao, Z., Shin, H. D., & Chen, R. (2009). A recombinant E. coli bioprocess for hyaluronan synthesis. Applied Microbiology and Biotechnology, 84(1), 63–69. doi:10.1007/s00253-009-1963-2.
Jin, P., Kang, Z., Yuan, P., Du, G., & Chen, J. (2016). Production of specific-molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168. Metabolic Engineering, 35, 21–30. doi:10.1016/j.ymben.2016.01.008.
Jia, Y., Zhu, J., Chen, X., Tang, D., Su, D., Yao, W., et al. (2013). Metabolic engineering of Bacillus subtilis for the efficient biosynthesis of uniform hyaluronic acid with controlled molecular weights. Bioresource Technology, 132, 427–431. doi:10.1016/j.biortech.2012.12.150.
Chauhan, A. S., Badle, S. S., Ramachandran, K. B., & Jayaraman, G. (2014). The P170 expression system enhances hyaluronan molecular weight and production in metabolically-engineered Lactococcus lactis. Biochemical Engineering Journal, 90, 73–78. doi:10.1016/j.bej.2014.05.012.
Yu, H., & Stephanopoulos, G. (2008). Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metabolic Engineering, 10(1), 24–32. doi:10.1016/j.ymben.2007.09.001.
Jeong, E., Shim, W. Y., & Kim, J. H. (2014). Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight. Journal of Biotechnology, 185, 28–36. doi:10.1016/j.jbiotec.2014.05.018.
Huang, W. C., Chen, S. J., & Chen, T. L. (2008). Production of hyaluronic acid by repeated batch fermentation. Biochemical Engineering Journal, 40(3), 460–464. doi:10.1016/j.bej.2008.01.021.
Patil, K. P., Kamalja, K. K., & Chaudhari, B. L. (2011). Optimization of medium components for hyaluronic acid production by Streptococcus zooepidemicus MTCC 3523 using a statistical approach. Carbohydrate Polymers, 86(4), 1573–1577. doi:10.1016/j.carbpol.2011.06.065.
Badle, S. S., Jayaraman, G., & Ramachandran, K. B. (2014). Ratio of intracellular precursors concentration and their flux influences hyaluronic acid molecular weight in Streptococcus zooepidemicus and recombinant Lactococcus lactis. Bioresource Technology, 163, 222–227. doi:10.1016/j.biortech.2014.04.027.
Tao, L., Song, F., Xu, N., Li, D., Linhardt, R. J., & Zhang, Z. (2017). New insights into the action of bacterial chondroitinase AC I and hyaluronidase on hyaluronic acid. Carbohydrate Polymers, 158, 85–92. doi:10.1016/j.carbpol.2016.12.010.
Hynes, W. L., & Walton, S. L. (2000). Hyaluronidases of gram-positive bacteria. FEMS Microbiology Letters, 183(2), 201–207. doi:10.1016/S0378-1097(99)00669-2.
Maniatis, T., Fritsch, E. F., & Sambrook, J. (1982). Molecular cloning: A laboratory manual (Vol. 545). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Chen, W. Y., Marcellin, E., Hung, J., & Nielsen, L. K. (2009). Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus. Journal of Biological Chemistry, 284(27), 18007–18014. doi:10.1074/jbc.M109.011999.
Bitter, T., & Muir, H. M. (1962). A modified uronic acid carbazole reaction. Analytical Biochemistry, 4, 330–334. doi:10.1016/0003-2697(62)90095-7.
Hynes, W. L., Ferretti, J. J., & Hynes, W. L. (1994). Assays for hyaluronidase activity. Methods in Enzymology, 235, 606–616.
Holden, M. T. G., Heather, Z., Paillot, R., Steward, K. F., Webb, K., Ainslie, F., et al. (2009). Genomic evidence for the evolution of Streptococcus equi host restriction, increased virulence, and genetic exchange with human pathogens. PLoS Pathogens. doi:10.1371/journal.ppat.1000346.
Hong-jie, F., Fu-yu, T., Ying, M., & Cheng-ping, L. (2009). Virulence and antigenicity of the szp-gene deleted Streptococcus equi ssp. zooepidemicus mutant in mice. Vaccine, 27(1), 56–61. doi:10.1016/j.vaccine.2008.10.037.
Liu, L., Du, G., Chen, J., Wang, M., & Sun, J. (2008). Influence of hyaluronidase addition on the production of hyaluronic acid by batch culture of Streptococcus zooepidemicus. Food Chemistry, 110(4), 923–926. doi:10.1016/j.foodchem.2008.02.082.
Park, M. G., Jang, J. D., & Kang, W. K. (1996). Streptococcus zooepidemicus medium and process for preparing hyaluronic acid. Google Patents.
Starr, C. R., & Engleberg, N. C. (2006). Role of hyaluronidase in subcutaneous spread and growth of group A streptococcus. Infection and Immunity, 74(1), 40–48. doi:10.1128/IAI.74.1.40-48.2006.
Schanté, C. E., Zuber, G., Herlin, C., & Vandamme, T. F. (2011). Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydrate Polymers, 85(3), 469–489. doi:10.1016/j.carbpol.2011.03.019.
Im, J. H., Song, J. M., Kang, J. H., & Kang, D. J. (2009). Optimization of medium components for high-molecular-weight hyaluronic acid production by Streptococcus sp. ID9102 via a statistical approach. Journal of Industrial Microbiology and Biotechnology, 36(11), 1337–1344. doi:10.1007/s10295-009-0618-8.
Zakeri, A., Rasaee, M. J., & Pourzardosht, N. (2017). Enhanced hyluronic acid production in Streptococcus zooepidemicus by over expressing HasA and molecular weight control with Niscin and glucose. Biotechnology Reports. doi:10.1016/j.btre.2017.02.007.
Armstrong, D. C., & Johns, M. R. (1997). Culture conditions affect the molecular weight properties of hyaluronic acid produced by Streptococcus zooepidemicus. Applied and Environmental Microbiology, 63(7), 2759–2764.
Tian, X., Azpurua, J., Hine, C., Vaidya, A., Myakishev-Rempel, M., Ablaeva, J., et al. (2013). High-molecularmass hyaluronan mediates the cancer resistance of the naked mole rat. Nature, 499(7458), 346–349. doi:10.1038/nature12234.
Acknowledgement
The authors wish to thank Tarbiat Modares University of Tehran for supporting the conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pourzardosht, N., Rasaee, M.J. Improved Yield of High Molecular Weight Hyaluronic Acid Production in a Stable Strain of Streptococcus zooepidemicus via the Elimination of the Hyaluronidase-Encoding Gene. Mol Biotechnol 59, 192–199 (2017). https://doi.org/10.1007/s12033-017-0005-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-017-0005-z