Abstract
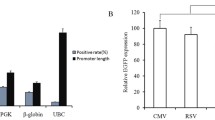
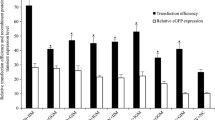
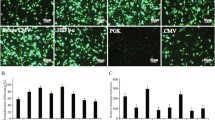
High expression level and long-term expression stability are required for therapeutic protein production in mammalian cells. Three commonly used promoters from the simian virus 40 (SV40), the CHO elongation factor 1α gene (EF1α), and the human cytomegalovirus major immediate early gene (CMV) and two matrix attachment regions from the chicken lysozyme gene (cMAR) and the human interferon β (iMAR) were evaluated for enhancing recombinant gene expression level and stability in stably transfected CHO cells. In the absence of MAR elements, the SV40 promoter gave lower expression level but higher stability than the EF1α promoter and the CMV promoter. The inclusion of MAR elements did not increase the integrated gene copies for all promoters but did enhance expression level for only the SV40 promoter. The enhanced gene expression was due to an increase in mRNA levels. Neither MAR elements enhance gene expression stability during long-term culture. The combinations of SV40 promoter and MAR elements are the best for obtaining both high expression level and stability. The information presented here would be valuable to those developing vectors for generation of CHO cell lines with stable and high productivity.




Similar content being viewed by others
References
Ho, S. C. L., Tong, Y. W., & Yang, Y. S. (2013). Generation of monoclonal antibody-producing mammalian cell lines. Pharmaceutical Bioprocessing, 1, 71–87.
Wurm, F. M. (2004). Production of recombinant protein therapeutics in cultivated mammalian cells. Nature Biotechnology, 22, 1393–1398.
Barnes, L. M., Bentley, C. M., & Dickson, A. J. (2001). Characterization of the stability of recombinant protein production in the GS-NS0 expression system. Biotechnology and Bioengineering, 73, 261–270.
Festenstein, R., Tolaini, M., Corbella, P., Mamalaki, C., Parrington, J., Fox, M., et al. (1996). Locus control region function and heterochromatin-induced position effect variegation. Science, 271, 1123–1125.
Goetze, S., Baer, A., Winkelmann, S., Nehlsen, K., Seibler, J., Maass, K., et al. (2005). Performance of genomic bordering elements at predefined genomic loci. Molecular and Cellular Biology, 25, 2260–2272.
Jordan, A., Defechereux, P., & Verdin, E. (2001). The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO Journal, 20, 1726–1738.
Rita Costa, A., Elisa Rodrigues, M., Henriques, M., Azeredo, J., & Oliveira, R. (2010). Guidelines to cell engineering for monoclonal antibody production. European Journal of Pharmaceutics and Biopharmaceutics, 74, 127–138.
Dorai, H., Corisdeo, S., Ellis, D., Kinney, C., Chomo, M., Hawley-Nelson, P., et al. (2012). Early prediction of instability of chinese hamster ovary cell lines expressing recombinant antibodies and antibody-fusion proteins. Biotechnology and Bioengineering, 109, 1016–1030.
Fann, C. H., Guirgis, F., Chen, G., Lao, M. S., & Piret, J. M. (2000). Limitations to the amplification and stability of human tissue-type plasminogen activator expression by Chinese hamster ovary cells. Biotechnology and Bioengineering, 69, 204–212.
He, L., Winterrowd, C., Kadura, I., & Frye, C. (2012). Transgene copy number distribution profiles in recombinant CHO cell lines revealed by single cell analyses. Biotechnology and Bioengineering, 109, 1713–1722.
Jun, S. C., Kim, M. S., Hong, H. J., & Lee, G. M. (2006). Limitations to the development of humanized antibody producing Chinese hamster ovary cells using glutamine synthetase-mediated gene amplification. Biotechnol. Progr., 22, 770–780.
Kim, M., O’Callaghan, P. M., Droms, K. A., & James, D. C. (2011). A mechanistic understanding of production instability in CHO cell lines expressing recombinant monoclonal antibodies. Biotechnology and Bioengineering, 108, 2434–2446.
Kim, S. J., Kim, N. S., Ryu, C. J., Hong, H. J., & Lee, G. M. (1998). Characterization of chimeric antibody producing CHO cells in the course of dihydrofolate reductase-mediated gene amplification and their stability in the absence of selective pressure. Biotechnology and Bioengineering, 58, 73–84.
Osterlehner, A., Simmeth, S., & Goepfert, U. (2011). Promoter methylation and transgene copy numbers predict unstable protein production in recombinant chinese hamster ovary cell lines. Biotechnology and Bioengineering, 108, 2670–2681.
Strutzenberger, K., Borth, N., Kunert, R., Steinfellner, W., & Katinger, H. (1999). Changes during subclone development and ageing of human antibody-producing recombinant CHO cells. Journal of Biotechnology, 69, 215–226.
Yang, Y., Mariati, Chusainow, J., & Yap, M. G. (2010). DNA methylation contributes to loss in productivity of monoclonal antibody-producing CHO cell lines. Journal of Biotechnology, 147, 180–185.
Deer, J. R., & Allison, D. S. (2004). High-level expression of proteins in mammalian cells using transcription regulatory sequences from the Chinese hamster EF-1 alpha gene. Biotechnology Progress, 20, 880–889.
Paredes, V., Park, J. S., Jeong, Y., Yoon, J., & Baek, K. (2013). Unstable expression of recombinant antibody during long-term culture of CHO cells is accompanied by histone H3 hypoacetylation. Biotechnology Letters, 35, 987–993.
Galbete, J. L., Bucetaz, M., & Mermod, N. (2009). MAR elements regulate the probability of epigenetic switching between active and inactive gene expression. Molecular Biosystems, 5, 143–150.
Byun, H. M., Suh, D. C., Jeong, Y. S., Wee, H. S., Kim, J. M., Kim, W. K., et al. (2005). Plasmid vectors harboring cellular promoters can induce prolonged gene expression in hematopoietic and mesenchymal progenitor cells. Biochemical and Biophysical Research Communications, 332, 518–523.
Damdindorj, L., Karnan, S., Ota, A., Takahashi, M., Konishi, Y., Hossain, E., et al. (2012). Assessment of the long-term transcriptional activity of a 550-bp-long human beta-actin promoter region. Plasmid, 68, 195–200.
Gill, D. R., Smyth, S. E., Goddard, C. A., Pringle, I. A., Higgins, C. F., Colledge, W. H., et al. (2001). Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1 alpha promoter. Gene Therapy, 8, 1539–1546.
Gopalkrishnan, R. V., Christiansen, K. A., Goldstein, N. I., DePinho, R. A., & Fisher, P. B. (1999). Use of the human EF-1 alpha promoter for expression can significantly increase success in establishing stable cell lines with consistent expression: a study using the tetracycline-inducible system in human cancer cells. Nucleic Acids Research, 27, 4775–4782.
Teschendorf, C., Warrington, K. H., Siemann, D. W., & Muzyczka, N. (2002). Comparison of the EF-1 alpha and the CMV promoter for engineering stable tumor cell lines using recombinant adeno-associated virus. Anticancer Research, 22, 3325–3330.
Chan, K. K. K., Wu, S. M., Nissom, P. M., Oh, S. K. W., & Choo, A. B. H. (2008). Generation of high-level stable transgene expressing human embryonic stem cell lines using Chinese hamster elongation factor-1 alpha promoter system. Stem Cells and Development, 17, 825–836.
Bode, J., Benham, C., Knopp, A., & Mielke, C. (2000). Transcriptional augmentation: Modulation of gene expression by scaffold/matrix-attached regions (S/MAR elements). Critical Reviews in Eukaryotic Gene, 10, 73–90.
Harraghy, N., Gaussin, A., & Mermod, N. (2008). Sustained transgene expression using MAR elements. Current Gene Therapy, 8, 353–366.
Araki, Y., Hamafuji, T., Noguchi, C., & Shimizu, N. (2012). Efficient recombinant production in mammalian cells using a novel IR/MAR gene amplification method. PLoS ONE, 7, e41787.
Kim, J. D., Yoon, Y., Hwang, H. Y., Park, J. S., Yu, S., Lee, J., et al. (2005). Efficient selection of stable Chinese hamster ovary (CHO) cell lines for expression of recombinant proteins by using human interferon beta SAR element. Biotechnology Progress, 21, 933–937.
Kim, J. M., Kim, J. S., Park, D. H., Kang, H. S., Yoon, J., Baek, K., et al. (2004). Improved recombinant gene expression in CHO cells using matrix attachment regions. Journal of Biotechnology, 107, 95–105.
Otte, A. P., Kwaks, T. H. J., van Blokland, R. J. M., Sewalt, R. G. A. B., Verhees, J., Klaren, V. N. A., et al. (2007). Various expression-augmenting DNA elements benefit from STAR-select, a novel high stringency selection system for protein expression. Biotechnology Progress, 23, 801–807.
Wang, F., Wang, T. Y., Tang, Y. Y., Zhang, J. H., & Yang, X. J. (2012). Different matrix attachment regions flanking a transgene effectively enhance gene expression in stably transfected Chinese hamster ovary cells. Gene, 500, 59–62.
Wang, T. Y., Yang, R., Qin, C. A., Wang, L., & Yang, X. J. (2008). Enhanced expression of transgene in CHO cells using matrix attachment region. Cell Biology International, 32, 1279–1283.
Wang, T. Y., Zhang, J. H., Jing, C. Q., Yang, X. J., & Lin, J. T. (2010). Positional effects of the matrix attachment region on transgene expression in stably transfected CHO cells. Cell Biology International, 34, 141–145.
Zahn-Zabal, M., Kobr, M., Girod, P. A., Imhof, M., Chatellard, P., de Jesus, M., et al. (2001). Development of stable cell lines for production or regulated expression using matrix attachment regions. Journal of Biotechnology, 87, 29–42.
Girod, P. A., Nguyen, D. Q., Calabrese, D., Puttini, S., Grandjean, M., Martinet, D., et al. (2007). Genome-wide prediction of matrix attachment regions that increase gene expression in mammalian cells. Nature Methods, 4, 747–753.
Girod, P. A., Zahn-Zabal, M., & Mermod, N. (2005). Use of the chicken lysozyme 5′ matrix attachment region to generate high producer CHO cell lines. Biotechnology and Bioengineering, 91, 1–11.
Dang, Q., Auten, J., & Plavec, I. (2000). Human beta interferon scaffold attachment region inhibits de novo methylation and confers long-term, copy number-dependent expression to a retroviral vector. Journal of Virology, 74, 2671–2678.
Moreno, R., Martinez, I., Petriz, J., Nadal, M., Tintore, X., Gonzalez, J. R., et al. (2011). The beta-interferon scaffold attachment region confers high-level transgene expression and avoids extinction by epigenetic modifications of integrated provirus in adipose tissue-derived human mesenchymal stem cells. Tissue Engineering Part C: Methods, 17, 275–287.
Ho, S. C. L., Bardor, M., Feng, H. T., Mariati, Tong, Y. W., Song, Z. W., et al. (2012). IRES-mediated Tricistronic vectors for enhancing generation of high monoclonal antibody expressing CHO cell lines. Journal of Biotechnology, 157, 130–139.
Piechaczek, C., Fetzer, C., Baiker, A., Bode, J., & Lipps, H. J. (1999). A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Research, 27, 426–428.
Chusainow, J., Yang, Y. S., Yeo, J. H., Toh, P. C., Asvadi, P., Wong, N. S., et al. (2009). A study of monoclonal antibody-producing CHO cell lines: what makes a stable high producer? Biotechnology and Bioengineering, 102, 1182–1196.
Sautter, K., & Enenkel, B. (2005). Selection of high-producing CHO cells using NPT selection marker with reduced enzyme activity. Biotechnology and Bioengineering, 89, 530–538.
Ng, S. K., Wang, D. I. C., & Yap, M. G. S. (2007). Application of destabilizing sequences on selection marker for improved recombinant protein productivity in CHO-DG44. Metabolic Engineering, 9, 304–316.
Bailey, L. A., Hatton, D., Field, R., & Dickson, A. J. (2012). Determination of Chinese hamster ovary cell line stability and recombinant antibody expression during long-term culture. Biotechnology and Bioengineering, 109, 2093–2103.
Senigl, F., Plachy, J., & Hejnar, J. (2008). The core element of a CpG island protects avian sarcoma and leukosis virus-derived vectors from transcriptional silencing. Journal of Virology, 82, 7818–7827.
Swindle, C. S., Kim, H. G., & Klug, C. A. (2004). Mutation of CpGs in the murine stem cell virus retroviral vector long terminal repeat represses silencing in embryonic stem cells. Journal of Biological Chemistry, 279, 34–41.
Harraghy, N., Regamey, A., Girod, P. A., & Mermod, N. (2011). Identification of a potent MAR element from the mouse genome and assessment of its activity in stable and transient transfections. Journal of Biotechnology, 154, 11–20.
Kalwy, S. (2005) Towards stronger gene expression: A promoter’s tale, In Presentation done at: 19th European Society for Animal Cell Technology (ESACT) meeting, Harrogate, England.
Acknowledgments
This work was supported by the Biomedical Research Council/Science and Engineering Research Council of A*STAR (Agency for Science, Technology and Research), Singapore.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ho, S.C.L., Mariati, Yeo, J.H.M. et al. Impact of Using Different Promoters and Matrix Attachment Regions on Recombinant Protein Expression Level and Stability in Stably Transfected CHO Cells. Mol Biotechnol 57, 138–144 (2015). https://doi.org/10.1007/s12033-014-9809-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9809-2