Abstract
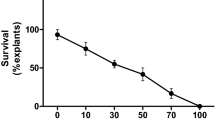
Bananas and plantains (Musa spp. L.) are important subsistence crops and premium export commodity in several countries, and susceptible to a wide range of environmental and biotic stress conditions. Here, we report efficient, rapid, and reproducible Agrobacterium-mediated transformation and regeneration of an Indian niche cultivar of banana [M. acuminata cv. Matti (AA)]. Apical meristem-derived highly proliferative multiple shoot clump (MSC) explants were transformed with the Agrobacterium strain EHA105 harboring a binary vector pCAMBIA-1301 carrying hptII and uidA. Sequential agro-infiltration (10 min, 400 mmHg), infection (additional 35 min, Agrobacterium density A 600 = 0.8) and co-cultivation (18 h) regimen in 100 µM acetosyringone containing liquid medium were critical factors yielding high transformation efficiency (~81 %) corroborated by transient GUS expression assay. Stable transgenic events were recovered following two cycles of meristem initiation and selection on hygromycin containing medium. Histochemical GUS assay in several tissues of transgenic plants and molecular analyses confirmed stable integration and expression of transgene. The protocol described here allowed recovery of well-established putative transgenic plantlets in as little as 5 months. The transgenic banana plants could be readily acclimatized under greenhouse conditions, and were phenotypically similar to the wild-type untransformed control plants (WT). Transgenic plants overexpressing Salinity-Induced Pathogenesis-Related class 10 protein gene from Arachis hypogaea (AhSIPR10) in banana cv. Matti (AA) showed better photosynthetic efficiency and less membrane damage (P < 0.05) in the presence of NaCl and mannitol in comparison to WT plants suggesting the role of AhSIPR10 in better tolerance of salt stress and drought conditions.





Similar content being viewed by others
Abbreviations
- AhSIPR10 :
-
Arachis hypogaea salinity-induced PR class 10 gene
- ECS:
-
Embryogenic cell suspension
- MS:
-
Murashige and Skoog medium
- MSC:
-
Multiple shoot clump
- PR10:
-
Pathogenesis-related (class 10)
- WT:
-
Wild-type
References
Aquil, B., Jan, A. T., Sarin, N. B., & Haq, Q. M. R. (2012). Micropropagation and genetic transformation of banana for crop improvement and sustainable agriculture. Journal of Crop Science, 3, 64–77.
D’Hont, A., Paget-Goy, A., Escoute, J., & Carreel, F. (2000). The interspecific genome structure of cultivated banana, Musa spp., revealed by genomic DNA in situ hybridization. Theoretical and Applied Genetics, 100, 177–183.
Heslop-Harrison, J. S., & Schwarzacher, T. (2007). Domestication, genomics and future for banana. Annals of Botany, 100, 1073–1084.
May, G. D., Afza, R., Mason, H. S., Wiecko, A., Novak, F. J., & Arntzen, C. J. (1995). Generation of transgenic banana (Musa acuminata) plants via Agrobacterium-mediated transformation. Nature Biotechnology, 13, 486–492.
Khanna, H., Becker, D., Kleidon, J., & Dale, J. (2004). Centrifugation assisted Agrobacterium tumefaciens-mediated transformation (CAAT) of embryogenic cell suspensions of banana (Musa spp. Cavendish AAA and Lady finger AAB). Molecular Breeding, 14, 239–252.
Tripathi, L., Tripathi, J. N., & Tushemereirwe, W. K. (2008). Rapid and efficient production of transgenic East African Highland Banana (Musa spp.) using intercalary meristematic tissues. African Journal of Biotechnology, 10, 1438–1445.
Ghosh, A., Ganapathi, T. R., Nath, P., & Bapat, V. A. (2009). Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in an important Cavendish banana cv. Robusta (AAA). Plant Cell, Tissue and Organ Culture, 97, 131–139.
Subramanyam, K., Subramanyam, K., Sailaja, K. V., Srinivasulu, M., & Lakshmidevi, K. (2011). Highly efficient Agrobacterium-mediated transformation of banana cv. Rasthali (AAB) via sonication and vacuum infiltration. Plant Cell Reports, 30, 425–436.
Chong-Pérez, B., Reyes, M., Rojas, L., Ocaña, B., Pérez, B., Kosky, R. G., et al. (2012). Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in banana cv. ‘Dwarf Cavendish’ (Musa AAA): effect of spermidine on transformation efficiency. Plant Cell, Tissue and Organ Culture, 111, 79–90.
Gilbert, R. A., Glynn, N. C., Comstock, J. C., & Davis, M. J. (2009). Agronomic performance and genetic characterization of sugarcane transformed for resistance to sugarcane yellow leaf virus. Field Crops Research, 111, 39–46.
Sreeramanan, S., Maziah, M., Rosli, N. M., Sariah, M., & Xavier, R. (2006). Enhanced tolerance against a fungal pathogen, Fusarium oxysporum f. sp. cubense (Race 1) in transgenic silk banana. International Journal of Agricultural Research, 4, 342–354.
Liu, J. J., & Ekramoddoullah, A. K. M. (2006). The family 10 of plant pathogenesis-related proteins: Their structure, regulation, and function in response to biotic and abiotic stresses. Physiological and Molecular Plant Pathology, 68, 3–13.
Kovács, G., Sági, L., Jacon, G., Arinaitwe, G., Busogoro, J. P., Thiry, E., et al. (2012). Expression of a rice chitinase gene in transgenic banana (‘Gros Michel’, AAA genome group) confers resistance to black leaf streak disease. Transgenic Research, 22, 117–130.
Ghag, S. B., Shekhawat, U. K. S., & Ganapathi, T. R. (2012). Petunia floral defensins with unique prodomains as novel candidates for development of Fusarium wilt resistance in transgenic banana plants. PLoS One, 7(6), e39557.
Xie, Y. R., Chen, Z. Y., Brown, R. L., & Bhatnagar, D. (2010). Expression and functional characterization of two pathogenesis-related protein 10 genes from Zea mays. Journal of Plant Physiology, 167, 121–130.
Choi, D. S., Hwang, I. S., & Hwang, B. K. (2012). Requirement of the cytosolic interaction between pathogenesis-related protein 10 and leucine-rich protein 1 for cell death and defense signaling in pepper. The Plant Cell, 24, 1675–1690.
Soh, H. C., Park, A. R., Park, S., Back, K., Yoon, J. B., Park, H. G., et al. (2012). Comparative analysis of pathogenesis-related protein 10 (PR10) genes between fungal resistant and susceptible peppers. European Journal of Plant Pathology, 132, 37–48.
Takeuchi, K., Gyohda, A., Tominaga, M., Kawakatsu, M., Hatakeyama, A., Ishii, N., et al. (2011). RSOsPR10 expression in response to environmental stresses is regulated antagonistically by jasmonate/ethylene and salicylic acid signaling pathways in rice roots. Plant Cell Physiology, 52, 1686–1696.
Jain, S., Kumar, D., Jain, M., Chaudhary, P., Deswal, R., & Sarin, N. B. (2012). Ectopic overexpression of a salt stress-induced pathogenesis related class 10 protein (PR10) gene from peanut (Arachis hypogaea L.) affords broad spectrum abiotic stress tolerance in transgenic tobacco. Plant Cell, Tissue and Organ Culture, 109, 19–31.
Hanafy, M., El-Banna, A., Schumacher, H. M., Jacobsen, H. J., & Hassan, F. (2013). Enhanced tolerance to drought and salt stresses in transgenic faba bean (Vicia faba L.) plants by heterologous expression of the PR10a gene from potato. Plant Cell Reports, 32, 663–674.
Jain, S., Srivastava, S., Sarin, N. B., & Kav, N. N. V. (2006). Proteomics reveals elevated levels of PR10 proteins in saline-tolerant peanut (Arachis hypogaea) calli. Plant Physiology and Biochemistry, 44, 253–259.
Uma, S., Mustaffa, M. M., Saraswathi, M. S., & Durai, P. (2010). Exploitation of diploids in Indian banana breeding programs. Acta Horticulturae, 897, 215–223.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Jefferson, R. A. (1987). Assaying chimeric genes in plants: The GUS fusion system. Plant Molecular Biology Reporter, 5, 387–405.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24, 1–15.
Sairam, R. K., & Srivastava, G. C. (2002). Changes in antioxidant activity in subcellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Science, 162, 897–904.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Vishnevetsky, J., White, T. L, Jr, Palmateer, A. J., Flaishman, M., Cohen, Y., Elad, Y., et al. (2011). Improved tolerance toward fungal diseases in transgenic Cavendish banana (Musa spp. AAA group) cv. Grand Nain. Transgenic Research, 20, 61–72.
Shekhawat, U. K. S., & Ganapathi, T. R. (2013). MusaWRKY71 overexpression in banana plants leads to altered abiotic and biotic stress responses. PLoS One, 8(10), e75506. doi:10.1371/journal.pone.0075506.
Huang, X., Huang, X. L., Xiao, W., Zhao, J. T., Dai, X. M., Chen, Y. F., et al. (2007). Highly efficient Agrobacterium-mediated transformation of embryogenic cell suspensions of Musa acuminata cv. Mas (AA) via a liquid co-cultivation system. Plant Cell Reports, 26, 1755–1762.
Srivastava, S., Neil Emery, R. J., Kurepin, L. V., Reid, D. M., Fristensky, B., & Kav, N. N. V. (2006). Pea PR10.1 is a ribonuclease and its transgenic expression elevates cytokinin levels. Plant Growth Regulation, 49, 17–25.
Krishnaswamy, S. S., Srivastava, S., Mohammadi, M., Rahman, M. H., Deyholos, M. K., & Kav, N. N. (2008). Transcriptional profiling of pea ABR17 mediated changes in gene expression in Arabidopsis thaliana. BMC Plant Biology, 8, 91.
Chung, K. M., Igari, K., Uchida, N., & Tasaka, M. (2008). New perspectives on plants defense responses through modulation of developmental pathways. Molecules and Cells, 26, 107–112.
Rivero, R. M., Gimeno, J., Deynze, A. V., Walia, H., & Blumwald, E. (2010). Enhanced cytokinin synthesis in tobacco plants expressing P SARK :IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiology, 51, 1929–1941.
Acknowledgments
This research work was supported by Department of Biotechnology, India (Grant no BT/PR10231/AGR/02/555/2007) to NBS. A.R. acknowledges the financial support from University Grant Commission (UGC). The authors also wish to thank Dr. Anuradha Agarwal, and Dr. Kailash Chander Bansal, Director, National Bureau of Plant Genetic Resources, New Delhi, India for disease-free banana plants. Research in the laboratory of NBS is supported by U.G.C.-C.A.S., U.G.C.-R.N.W., Department of Science and Technology (D.S.T.)-F.I.S.T., and D.S.T.-PURSE.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rustagi, A., Jain, S., Kumar, D. et al. High Efficiency Transformation of Banana [Musa acuminata L. cv. Matti (AA)] for Enhanced Tolerance to Salt and Drought Stress Through Overexpression of a Peanut Salinity-Induced Pathogenesis-Related Class 10 Protein. Mol Biotechnol 57, 27–35 (2015). https://doi.org/10.1007/s12033-014-9798-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9798-1