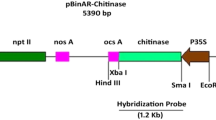
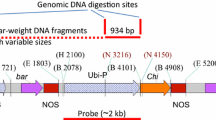
Abstract
Multi-auto-transformation vector system has been one of the strategies to produce marker-free transgenic plants without using selective chemicals and plant growth regulators and thus facilitating transgene stacking. In the study reported here, retransformation was carried out in marker-free transgenic potato CV. May Queen containing ChiC gene (isolated from Streptomyces griseus strain HUT 6037) with wasabi defensin (WD) gene (isolated from Wasabia japonica) to pyramid the two disease resistant genes. Molecular analyses of the developed shoots confirmed the existence of both the genes of interest (ChiC and WD) in transgenic plants. Co-expression of the genes was confirmed by RT-PCR, northern blot, and western blot analyses. Disease resistance assay of in vitro plants showed that the transgenic lines co-expressing both the ChiC and WD genes had higher resistance against the fungal pathogens, Fusarium oxysporum (Fusarium wilt) and Alternaria solani (early blight) compared to the non-transformed control and the transgenic lines expressing either of the ChiC or WD genes. The disease resistance potential of the transgenic plants could be increased by transgene stacking or multiple transformations.






Similar content being viewed by others
References
Collinge, D. B., Kragh, K. M., Mikkelsen, J. D., Nielsen, K. K., Rasmussen, U., & Vad, K. (1993). Plant chitinases. The Plant Journal, 3, 31–40.
Graham, L. S., & Sticklen, M. B. (1993). Plant chitinases. Canadian Journal of Botany, 72, 1057–1083.
Broglie, K., Chet, I., Holliday, M., Cressman, R., Biddle, P., Knowlton, S., et al. (1991). Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science, 254, 1194–1197.
Punja, Z. K., & Raharjo, S. H. T. (1996). Response of transgenic cucumber and carrot plants expressing different chitinase enzymes to inoculation with pathogens. Plant Diseases, 80, 999–1005.
Khan, R. S., Sjahril, R., Nakamura, I., & Mii, M. (2008). Production of transgenic potato exhibiting enhanced resistance to fungal infection and herbicide applications. Plant Biotechnology Reports, 2, 13–20.
Khan, R. S., Ntui, V. O., Chin, D. P., Nakamura, I., & Mii, M. (2011). Production of marker-free disease-resistant potato using isopentenyl transferase gene as a positive selection marker. Plant Cell Reports, 30, 587–597.
Pedras, M. S. C., Sorensen, J. I., Okanga, F. I., & Zaharia, I. (1999). Wasalexins A and B, new phytoalexins from wasabi: isolation and synthesis, and antifungal activity. Medical and Chemical Letters, 9, 3015–3020.
Saitoh, H., Kiba, A., Nishihara, M., Yamamura, S., Suzuki, K., & Terauchi, R. (2001). Production of antimicrobial defensin in Nicotiana benthamiana with a potato virus X vector. Molecular Plant-Microbe Interactions, 14, 111–115.
Kanzaki, H., Nirasawa, S., Saitoh, H., et al. (2002). Over expression of the wasabi defensin gene confers enhanced resistance to blast fungus (Magnaporthe grisea) in transgenic rice. Theoretical and Applied Genetics, 105, 809–814.
Khan, R. S., Nishihara, M., Yamamura, S., Nakamura, I., & Mii, M. (2006). Transgenic potatoes expressing wasabi defensin peptide confer partial resistance to gray mold (Botrytis cinerea). Plant Biotechnology, 23, 179–183.
Khan, R. S., Nakamura, I., & Mii, M. (2011). Development of disease-resistant marker-free tomato by R/RS site-specific recombination. Plant Cell Reports, 30, 1041–1053.
Zhang, S., Song, W. Y., Chen, L., Ruan, D., Taylor, N., Ronald, P. C., et al. (1998). Transgenic elite indica rice varieties, resistant to Xanthomonas oryzae pv. oryzae. Molecular Breeding, 4, 551–558.
Mew, T. W., Vera Cruz, C. M., & Medalla, E. S. (1992). Changes in the race frequency of Xanthomonas oryzae pv. oryzae in response to the planting of rice cultivars in the Philippines. Plant Diseases, 76, 1029–1032.
Huang, N., Angeles, E. R., Domingo, J., Magpantay, G., Singh, S., Zhang, G., et al. (1997). Pyramiding of bacterial blight resistance genes in rice: marker assisted selection using RFLP and PCR. Theoretical and Applied Genetics, 95, 313–320.
Velazhahan, R., Chen-Cole, K., Anuratha, C. S., & Muthukrishnan, S. (1998). Induction of thaumatin-like proteins (TLPs) in Rhizoctonia solani infected rice and characterization of two new cDNA clones. Physiologia Plantarum, 102, 21–28.
Takakura, Y., Ito, T., Saito, H., Inoue, T., Komari, T., & Kuwata, S. (2000). Flower predominant expression of a gene encoding a novel class I chitinase in rice (Oryza sativa L.). Plant Molecular Biology, 42, 883–897.
Terras, F. R. G., Eggernont, K., Kovaleva, V., et al. (1995). Small cysteinerich antifungal proteins from radish: their role in host defense. The Plant Cell, 7, 573–588.
Christo, P. (1997). Rice transformation: bombardment. Plant Molecular Biology, 35, 197–203.
Chen, L., Marmey, P., Taylor, N. J., Brizard, J. P., Espinoza, C., Cruz, P., et al. (1998). Expression and inheritance of multiple transgenes in rice plants. Nature Biotechnology, 16, 1060–1064.
Kim, J. K., Jang, I. C., Wu, R., Zuo, W. N., Boston, R. S., Lee, Y. H., et al. (2003). Coexpression of a modified maize ribosome-inactivating protein and a rice basic chitinase gene in transgenic rice plants confers enhanced resistance to sheath blight. Transgenic Research, 12, 475–484.
Wally, O., Jayaraj, J., & Punja, Z. (2009). Comparative resistance to foliar fungal pathogens in transgenic carrot plants expressing genes encoding for chitinase, β-1,3-glucanase and peroxidise. European Journal of Plant Pathology, 123, 331–342.
Jha, S., & Chattoo, B. B. (2009). Transgene stacking and coordinated expression of plant defensins confer fungal resistance in rice. Rice, 2, 143–154.
Mehrotra, M., Singh, A. K., Sanyal, I., Altosaar, I., & Amla, D. V. (2011). Pyramiding of modified cry1Ab and cry1Ac genes of Bacillus thuringiensis in transgenic chickpea (Cicer arietinum L.) for improved resistance to pod borer insect Helicoverpa armigera. Euphytica, 182, 87–102.
Liu, Z. W., Li, H. P., Cheng, W., Yang, P., Zhang, J. B., Gong, A. D., et al. (2012). Enhanced overall resistance to Fusarium seedling blight and Fusarium head blight in transgenic wheat by co-expression of anti-fungal peptides. European Journal of Plant Pathology, 134, 721–732.
He, C., He, Y., Liu, Q., Liu, T., Liu, C., Wang, L., et al. (2013). Co-expression of genes ApGSMT2 and ApDMT2 for glycinebetaine synthesis in maize enhances the drought tolerance of plants. Molecular Breeding, 31, 559–573.
Bezirganoglu, I., Hwang, S. Y., Fang, T. J., & Shaw, J. F. (2013). Transgenic lines of melon Cucumis melo L. var. makuwa cv. ‘Silver Light’) expressing antifungal protein and chitinase genes exhibit enhanced resistance to fungal pathogens. Plant Cell, Tissue and Organ Culture, 112, 227–237.
Ntui, V. O., Azadi, P., Thirukkumaran, G., Khan, R. S., Chin, D. P., Nakamura, I., et al. (2011). Increased resistance to fusarium wilt in transgenic tobacco lines co-expressing chitinase and wasabi defensin genes. Plant Pathology, 60, 221–231.
Ebinuma, H., Sugita, K., Matsunaga, E., & Yamakado, M. (1997). Selection of marker-free transgenic plants using the isopentenyl transferase gene as a selectable marker. Proceedings of National Academy of Sciences USA, 99, 2117–2121.
Khan, R. S., Nakamura, I., & Mii, M. (2010). Production and selection of marker-free transgenic plants of Petunia hybrida using site-specific recombination. Biologia Plantarum, 54, 265–271.
Sugita, K., Matsunaga, E., Kasahara, T., & Ebinuma, H. (2000). Transgene stacking in plants in the absence of sexual crossing. Molecular Breeding, 6, 529–536.
Khan, R. S., Chin, D. P., Nakamura, I., & Mii, M. (2006). Production of marker-free Nierembergia caerulea using MAT vector system. Plant Cell Reports, 25, 914–919.
Araki, H., Jearnpipatkula, A., Tatsumi, H., Sakurai, T., Ushino, K., Muta, T., et al. (1987). Molecular and functional organization of yeast plasmid pSR1. Journal of Molecular Biology, 182, 191–203.
Smigocki, A. C. (1991). Cytokinin content and tissue distribution in plants transformed by a reconstructed isopentenyl transferase gene. Plant Molecular Biology, 16, 105–115.
Hewelt, A., Prinsen, E., Schell, J., Van Onckelen, H., & Schmuülling, T. (1994). Promoter tagging with a promoterless ipt gene leads to cytokinin-induced phenotypic variability in transgenic tobacco plants: implications of gene dosage effects. Plant Journal, 6, 879–891.
Walter, R. S., Rosemary, L., Gary, D. F., & Weingartner, D. P. (2001). Compendium of potato diseases (APS compendium of plant disease series). St Paul: American Phytopathological Society.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Rogers, O. S., & Bendich, J. A. (1988). Extraction of DNA from plant tissues. In S. B. Gelvin, R. A. Schiliperoort, & D. P. S. Verma (Eds.), Plant Molecular Biology Manual (Vol. A6, pp. 1–10). Dordrecht: Kluwer Academic Publishers.
Sambrook, J., & Russell, D. W. (2001). Molecular cloning. A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Broekaert, W. F., Cammue, B. P. A., De Bolle, M. F. C., Thevissen, K., de Samblanx, G. W., & Osborn, R. W. (1997). Antimicrobial peptides from plants. Critical Review of Plant Science, 16, 297–323.
Ntui, V. O., Thirukkumaran, G., Azadi, P., Khan, R. S., Nakamura, I., & Mii, M. (2010). Stable integration and expression of wasabi defensin gene in ‘‘Egusi’’ melon (Colocynthis citrullus L.) confers resistance to Fusarium wilt and Alternaria leaf spot. Plant Cell Reports, 29, 943–954.
Chhikara, S., Chaudhury, D., Dhankher, O. P., & Jaiwal, P. K. (2012). Combined expression of a barley class II chitinase and type I ribosome inactivating protein in transgenic Brassica juncea provides protection against Alternaria brassicae. Plant Cell, Tissue and Organ Culture, 108, 83–89.
Gao, A. G., Hakimi, S. M., Mittanck, C. A., et al. (2000). Fungal pathogen protection in potato by expression of a plant defensin peptide. Nature Biotechnology, 18, 1307–1310.
Leah, R., Tommerrup, H., Svendson, I., & Mundy, J. (1991). Biochemical and molecular characterization of three barley seed proteins with anti-fungal properties. Journal of Biological Chemistry, 266, 1564–1573.
Jongedijk, E., Tigelaar, H., Van Roekel, J. S. C., Bres-Vloemans, S. A., Dekker, I., Van den Elzen, P. J. M., et al. (1995). Synergistic activity of chitinases and b-1,3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica, 85, 173–180.
Chen, S. C., Liu, A. R., Wang, F. H., & Ahammed, G. J. (2009). Combined overexpression of chitinase and defensin genes in transgenic tomato enhances resistance to Botrytis cinerea. African Journal of Biotechnology, 8, 5182–5188.
Acknowledgments
We are very grateful to Pulp and Paper Research group, Nippon Paper Industries, Tokyo, who kindly provided the MAT vector constructs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, R.S., Darwish, N.A., Khattak, B. et al. Retransformation of Marker-Free Potato for Enhanced Resistance Against Fungal Pathogens by Pyramiding Chitinase and Wasabi Defensin Genes. Mol Biotechnol 56, 814–823 (2014). https://doi.org/10.1007/s12033-014-9760-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9760-2