Abstract
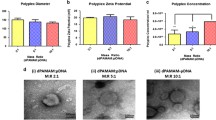
Hydrogels are increasingly being investigated as a means to implant cells for tissue engineering. One way to further enhance the repair response would be to combine the hydrogel cell carrier with gene transfer. Gene therapy, using adenoviral vectors, is an effective way to provide transient delivery of bioactive factors. However, current protocols require further optimization, especially if they are to be transferred into the clinic. This study opted to compare the efficiency of protocols for standard two-dimensional (2D) versus three-dimensional (3D), adenoviral-mediated, transduction of human mesenchymal stem cells. Two different multiplicities of infection were tested. After encapsulation in fibrin, alginate or agarose, cells were cultured for 28 days. Transduction in 3D showed a much higher efficiency, compared to standard 2D transduction protocols. In 3D, the amount of transgene produced was significantly higher, for every condition investigated. Furthermore, transduction in 3D does not require a cell culture step and can be conducted within the operating theatre. In conclusion, it was demonstrated that 3D transduction, using adenoviral vectors, is superior to standard transduction protocols in 2D. It therefore, might help increasing its administration in tissue engineering and clinical applications.




Similar content being viewed by others
References
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260, 920–926.
Zuk, P. A., Zhu, M., Ashjian, P., De Ugarte, D. A., Huang, J. I., Mizuno, H., et al. (2002). Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell, 13, 4279–4295.
Cao, B., Zheng, B., Jankowski, R. J., Kimura, S., Ikezawa, M., Deasy, B., et al. (2003). Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nature Cell Biology, 5, 640–646.
De Bari, C., Dell’Accio, F., Tylzanowski, P., & Luyten, F. P. (2001). Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and Rheumatism, 44, 1928–1942.
Friedenstein, A. J., Gorskaja, J. F., & Kulagina, N. N. (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology, 4, 267–274.
Ashton, B. A., Allen, T. D., Howlett, C. R., Eaglesom, C. C., Hattori, A., & Owen, M. (1980). Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clinical Orthopaedics and Related Research 151, 294–307.
Johnstone, B., Hering, T. M., Caplan, A. I., Goldberg, V. M., & Yoo, J. U. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research, 238, 265–272.
Urist, M. R. (1965). Bone: Formation by autoinduction. Science, 150, 893–899.
Gooch, K. J., Blunk, T., Courter, D. L., Sieminski, A. L., Vunjak-Novakovic, G., & Freed, L. E. (2002). Bone morphogenetic proteins-2, -12, and -13 modulate in vitro development of engineered cartilage. Tissue Engineering, 8, 591–601.
Sekiya, I., Larson, B. L., Vuoristo, J. T., Reger, R. L., & Prockop, D. J. (2005). Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell and Tissue Research, 320, 269–276.
Sellers, R. S., Zhang, R., Glasson, S. S., Kim, H. D., Peluso, D., D’Augusta, D. A., et al. (2000). Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2). Journal of Bone and Joint Surgery, 82, 151–160.
Uusitalo, H., Hiltunen, A., Ahonen, M., Kahari, V. M., Aro, H., & Vuorio, E. (2001). Induction of periosteal callus formation by bone morphogenetic protein-2 employing adenovirus-mediated gene delivery. Matrix Biology, 20, 123–127.
Virk, M. S., Conduah, A., Park, S. H., Liu, N., Sugiyama, O., Cuomo, A., et al. (2008). Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone, 42, 921–931.
Gelse, K., von der, M. K., Aigner, T., Park, J., & Schneider, H. (2003). Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis and Rheumatism, 48, 430–441.
Palmer, G. D., Steinert, A., Pascher, A., Gouze, E., Gouze, J. N., Betz, O., et al. (2005). Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Molecular Therapy, 12, 219–228.
Steinert, A. F., Palmer, G. D., Pilapil, C., Noth, U., Evans, C. H., & Ghivizzani, S. C. (2009). Enhanced in vitro chondrogenesis of primary mesenchymal stem cells by combined gene transfer. Tissue Engineering Part A, 15, 1127–1139.
Steinert, A., Weber, M., Dimmler, A., Julius, C., Schutze, N., Noth, U., et al. (2003). Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate. Journal of Orthopaedic Research, 21, 1090–1097.
Weisser, J., Rahfoth, B., Timmermann, A., Aigner, T., Brauer, R., & von der, M. K. (2001). Role of growth factors in rabbit articular cartilage repair by chondrocytes in agarose. Osteoarthritis Cartilage, 9(Suppl A), S48–S54.
Ahmed, T. A., Dare, E. V., & Hincke, M. (2008). Fibrin: A versatile scaffold for tissue engineering applications. Tissue Engineering Part B Reviews, 14, 199–215.
Pelaez, D., Huang, C. Y., & Cheung, H. S. (2009). Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells and Development, 18, 93–102.
Eyrich, D., Wiese, H., Maier, G., Skodacek, D., Appel, B., Sarhan, H., et al. (2007). In vitro and in vivo cartilage engineering using a combination of chondrocyte-seeded long-term stable fibrin gels and polycaprolactone-based polyurethane scaffolds. Tissue Engineering, 13, 2207–2218.
Grad, S., Kupcsik, L., Gorna, K., Gogolewski, S., & Alini, M. (2003). The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: Potential and limitations. Biomaterials, 24, 5163–5171.
Li, Z., Kupcsik, L., Yao, S. J., Alini, M., & Stoddart, M. J. (2009). Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin–polyurethane composites. Tissue Engineering Part A, 15, 1729–1737.
Visna, P., Pasa, L., Cizmar, I., Hart, R., & Hoch, J. (2004). Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques–a randomized controlled study. Acta Chirurgica Belgica, 104, 709–714.
Gouze, E., Pawliuk, R., Pilapil, C., Gouze, J. N., Fleet, C., Palmer, G. D., et al. (2002). In vivo gene delivery to synovium by lentiviral vectors. Molecular Therapy, 5, 397–404.
Nita, I., Ghivizzani, S. C., Galea-Lauri, J., Bandara, G., Georgescu, H. I., Robbins, P. D., et al. (1996). Direct gene delivery to synovium. An evaluation of potential vectors in vitro and in vivo. Arthritis and Rheumatism, 39, 820–828.
Roessler, B. J., Allen, E. D., Wilson, J. M., Hartman, J. W., & Davidson, B. L. (1993). Adenoviral-mediated gene transfer to rabbit synovium in vivo. Journal of Clinical Investigation, 92, 1085–1092.
Kang, R., Marui, T., Ghivizzani, S. C., Nita, I. M., Georgescu, H. I., Suh, J. K., et al. (1997). Ex vivo gene transfer to chondrocytes in full-thickness articular cartilage defects: A feasibility study. Osteoarthritis Cartilage, 5, 139–143.
Mason, J. M., Grande, D. A., Barcia, M., Grant, R., Pergolizzi, R. G., & Breitbart, A. S. (1998). Expression of human bone morphogenic protein 7 in primary rabbit periosteal cells: Potential utility in gene therapy for osteochondral repair. Gene Therapy, 5, 1098–1104.
Dinser, R., Kreppel, F., Zaucke, F., Blank, C., Paulsson, M., Kochanek, S., et al. (2001). Comparison of long-term transgene expression after non-viral and adenoviral gene transfer into primary articular chondrocytes. Histochemistry and Cell Biology, 116, 69–77.
Steinert, A. F., Noth, U., & Tuan, R. S. (2008). Concepts in gene therapy for cartilage repair. Injury, 39(Suppl 1), S97–S113.
Thomas, C. E., Ehrhardt, A., & Kay, M. A. (2003). Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics, 4, 346–358.
Hao, J., Yao, Y., Varshney, R. R., Wang, L., Prakash, C., Li, H., et al. (2008). Gene transfer and living release of transforming growth factor-beta3 for cartilage tissue engineering applications. Tissue Engineering Part C Methods, 14, 273–280.
Meinel, L., Hofmann, S., Betz, O., Fajardo, R., Merkle, H. P., Langer, R., et al. (2006). Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: Comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials, 27, 4993–5002.
Steinert, A. F., Proffen, B., Kunz, M., Hendrich, C., Ghivizzani, S. C., Noth, U., et al. (2009). Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis Research and Therapy, 11, R148.
Danthinne, X., & Imperiale, M. J. (2000). Production of first generation adenovirus vectors: A review. Gene Therapy, 7, 1707–1714.
Peng, Z. (2005). Current status of gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Human Gene Therapy, 16, 1016–1027.
Penny, W. F., & Hammond, H. K. (2004). Clinical use of intracoronary gene transfer of fibroblast growth factor for coronary artery disease. Current Gene Therapy, 4, 225–230.
Gelse, K., Jiang, Q. J., Aigner, T., Ritter, T., Wagner, K., Poschl, E., von der, M. K., and Schneider, H. (2001). Fibroblast-mediated delivery of growth factor complementary DNA into mouse joints induces chondrogenesis but avoids the disadvantages of direct viral gene transfer. Arthritis and Rheumatism, 44, 1943–1953.
Zachos, T., Diggs, A., Weisbrode, S., Bartlett, J., & Bertone, A. (2007). Mesenchymal stem cell-mediated gene delivery of bone morphogenetic protein-2 in an articular fracture model. Molecular Therapy, 15, 1543–1550.
Ho, S. T., Cool, S. M., Hui, J. H., & Hutmacher, D. W. (2010). The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials, 31, 38–47.
Huang, C. Y., Reuben, P. M., D’Ippolito, G., Schiller, P. C., & Cheung, H. S. (2004). Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. The Anatomical Record Part A Discoveries in Molecular, Cellular, and Evolutionary Biology, 278, 428–436.
Li, Z., Yao, S. J., Alini, M., & Stoddart, M. J. (2009). Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Engineering Part A 16(2), 575–584.
He, T. C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W., & Vogelstein, B. (1998). A simplified system for generating recombinant adenoviruses. Proceedings of the National Academy of Sciences of USA, 95, 2509–2514.
Kupcsik, L., Stoddart, M. J., Li, Z., Benneker, L. M., & Alini, M. (2010). Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Engineering Part A, 16, 1845–1855.
Kupcsik, L., Alini, M., & Stoddart, M. J. (2009). Epsilon-aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Engineering Part A, 15, 2309–2313.
Schek, R. M., Hollister, S. J., & Krebsbach, P. H. (2004). Delivery and protection of adenoviruses using biocompatible hydrogels for localized gene therapy. Molecular Therapy, 9, 130–138.
Teraishi, F., Umeoka, T., Saito, T., Tsukagoshi, T., Tanaka, N., & Fujiwara, T. (2003). A novel method for gene delivery and expression in esophageal epithelium with fibrin glues containing replication-deficient adenovirus vector. Surgical Endoscopy, 17, 1845–1848.
Breen, A., Dockery, P., O’Brien, T., & Pandit, A. (2009). Fibrin scaffold promotes adenoviral gene transfer and controlled vector delivery. Journal of Biomedical Materials Research Part A, 89, 876–884.
Breen, A. M., Dockery, P., O’Brien, T., & Pandit, A. S. (2008). The use of therapeutic gene eNOS delivered via a fibrin scaffold enhances wound healing in a compromised wound model. Biomaterials, 29, 3143–3151.
Olmsted-Davis, E. A., Gugala, Z., Gannon, F. H., Yotnda, P., McAlhany, R. E., Lindsey, R. W., et al. (2002). Use of a chimeric adenovirus vector enhances BMP2 production and bone formation. Human Gene Therapy, 13, 1337–1347.
Shayakhmetov, D. M., Papayannopoulou, T., Stamatoyannopoulos, G., & Lieber, A. (2000). Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. Journal of Virology, 74, 2567–2583.
Majumdar, M. K., Wang, E., & Morris, E. A. (2001). BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. Journal of Cellular Physiology, 189, 275–284.
Sailor, L. Z., Hewick, R. M., & Morris, E. A. (1996). Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. Journal of Orthopaedic Research, 14, 937–945.
Toh, W. S., Yang, Z., Liu, H., Heng, B. C., Lee, E. H., & Cao, T. (2007). Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells, 25, 950–960.
Morral, N., O’Neal, W. K., Rice, K., Leland, M. M., Piedra, P. A., Aguilar-Cordova, E., et al. (2002). Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Human Gene Therapy, 13, 143–154.
Thomas, C. E., Birkett, D., Anozie, I., Castro, M. G., & Lowenstein, P. R. (2001). Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Molecular Therapy, 3, 36–46.
Pascher, A., Palmer, G. D., Steinert, A., Oligino, T., Gouze, E., Gouze, J. N., et al. (2004). Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Therapy, 11, 133–141.
He, C. X., Li, N., Hu, Y. L., Zhu, X. M., Li, H. J., Han, M., et al. (2011). Effective gene delivery to mesenchymal stem cells based on the reverse transfection and three-dimensional cell culture system. Pharmaceutical Research, 28, 1577–1590.
Aviles, M. O., & Shea, L. D. (2011). Hydrogels to modulate lentivirus delivery in vivo from microporous tissue engineering scaffolds. Drug Delivery and Translational Research, 1, 91–101.
Acknowledgments
The fibrin used in the experiments was generously supplied by Baxter Biosurgery (Vienna, Austria). This study was supported by Swiss National Fund [SNF 320000-116846/1].
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neumann, A.J., Schroeder, J., Alini, M. et al. Enhanced Adenovirus Transduction of hMSCs Using 3D Hydrogel Cell Carriers. Mol Biotechnol 53, 207–216 (2013). https://doi.org/10.1007/s12033-012-9522-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9522-y