Abstract
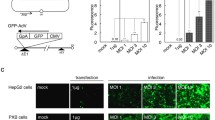
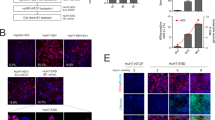
Although much has been learned about Hepatitis C virus (HCV), research progress has been hindered by the lack of a suitable cell culture system supporting its replication. Recently, a unique HCV strain JFH1 has been found to replicate efficiently in cell culture with production of infectious HCV (HCVcc). Baculovirus vectors were found to be efficient delivery vehicles and a HBV recombinant baculovirus/HepG2 system efficiently delivered the HBV genome into HepG2 resulting in HBV replication. In this study, we developed a recombinant baculovirus expression system to generate infectious HCV particles in hepatoma cell line Huh7-lunetT7 by using cDNA from the HCV JFH1 genotype. Results show that HCV positive, negative RNA strands and proteins were produced in this system. Furthermore, HCV particles were produced and secreted into the culture medium. Sucrose density gradient centrifugation of the culture medium revealed co-localization of HCV RNA and structural proteins in the fraction with a density of 1.08–1.13 g/ml. Electron microscopy (EM) showed viral particles approximately 55 nm in diameter, which could be recognized by anti-HCV E2 antibodies. Real-time RT-PCR detected that the level of HCV vRNA in the supernatant was 107 copies/ml at 72 h post-transduction (hpt). In addition, the JFH1 virus produced by the recombinant baculovirus was confirmed to be infectious in vitro. In summary, this system provides a novel tool not only for the analysis of the replication and pathogenesis of HCV but also to screen for potential therapeutic targets.







Similar content being viewed by others
References
Abdelhamed, A. M., Kelley, C. M., Miller, T. G., Furman, P. A., & Isom, H. C. (2002). Rebound of hepatitis B virus replication in HepG2 cells after cessation of antiviral treatment. Journal of Virology, 76, 8148–8160. doi:10.1128/JVI.76.16.8148-8160.2002.
Ball, L. A. (1994). Replication of the genomic RNA of a positive-strand RNA animal virus from negative-sense transcripts. Proceedings of the National Academy of Sciences of the United States of America, 91, 12443–12447. doi:10.1073/pnas.91.26.12443.
Baumert, T. F., Vergalla, J., Satoi, J., Thomson, M., Lechmann, M., Herion, D., et al. (1999). Hepatitis C virus-like particles synthesized in insect cells as a potential vaccine candidate. Gastroenterology, 117, 1397–1407. doi:10.1016/S0016-5085(99)70290-8.
Blight, K. J., McKeating, J. A., Marcotrigiano, J., & Rice, C. M. (2003). Efficient replication of hepatitis C virus genotype 1a RNAs in cell culture. Journal of Virology, 77, 3181–3190. doi:10.1128/JVI.77.5.3181-3190.2003.
Blight, K. J., McKeating, J. A., & Rice, C. M. (2002). Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. Journal of Virology, 76, 13001–13014. doi:10.1128/JVI.76.24.13001-13014.2002.
Cai, Z., Zhang, C., Chang, K. S., Jiang, J., Ahn, B. C., Wakita, T., et al. (2005). Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. Journal of Virology, 79, 13963–13973. doi:10.1128/JVI.79.22.13963-13973.2005.
Chen, C., & Okayama, H. (1987). High-efficiency transformation of mammalian cells by plasmid DNA. Molecular and Cellular Biology, 7, 2745–2752.
Chung, R. T., He, W., Saquib, A., Contreras, A. M., Xavier, R. J., Chawla, A., et al. (2001). Hepatitis C virus replication is directly inhibited by IFN-alpha in a full-length binary expression system. Proceedings of the National Academy of Sciences of the United States of America, 98, 9847–9852. doi:10.1073/pnas.171319698.
Craggs, J. K., Ball, J. K., Thomson, B. J., Irving, W. L., & Grabowska, A. M. (2001). Development of a strand-specific RT-PCR based assay to detect the replicative form of hepatitis C virus RNA. Journal of Virological Methods, 94, 111–120. doi:10.1016/S0166-0934(01)00281-6.
Delaney, W. E., & Isom, H. C. (1998). Hepatitis B virus replication in human HepG2 cells mediated by hepatitis B virus recombinant baculovirus. Hepatology (Baltimore, MD.), 28, 1134–1146. doi:10.1002/hep. 510280432.
Fipaldini, C., Bellei, B., & La Monica, N. (1999). Expression of hepatitis C virus cDNA in human hepatoma cell line mediated by a hybrid baculovirus-HCV vector. Virology, 255, 302–311. doi:10.1006/viro.1998.9565.
Friebe, P., Boudet, J., Simorre, J. P., & Bartenschlager, R. (2005). Kissing-loop interaction in the 3′ end of the hepatitis C virus genome essential for RNA replication. Journal of Virology, 79, 380–392. doi:10.1128/JVI.79.1.380-392.2005.
Fuerst, T. R., Niles, E. G., Studier, F. W., & Moss, B. (1986). Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proceedings of the National Academy of Sciences of the United States of America, 83, 8122–8126. doi:10.1073/pnas.83.21.8122.
Heller, T., Saito, S., Auerbach, J., Williams, T., Moreen, T. R., Jazwinski, A., et al. (2005). An in vitro model of hepatitis C virion production. Proceedings of the National Academy of Sciences of the United States of America, 102, 2579–2583. doi:10.1073/pnas.0409666102.
Hiramatsu, N., Dash, S., & Gerber, M. A. (1997). HCV cDNA transfection to HepG2 cells. Journal of Viral Hepatitis, 4(Suppl 1), 61–67. doi:10.1111/j.1365-2893.1997.tb00162.x.
Hoofnagle, J. H. (2002). Course and outcome of hepatitis C. Hepatology (Baltimore, Md.), 36, S21–S29. doi:10.1002/hep.1840360704.
Kaito, M., Watanabe, S., Tsukiyama-Kohara, K., Yamaguchi, K., Kobayashi, Y., Konishi, M., et al. (1994). Hepatitis C virus particle detected by immunoelectron microscopic study. The Journal of General Virology, 75(Pt 7), 1755–1760.
Kato, T., Date, T., Miyamoto, M., Furusaka, A., Tokushige, K., Mizokami, M., et al. (2003). Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology, 125, 1808–1817. doi:10.1053/j.gastro.2003.09.023.
Kato, T., Matsumura, T., Heller, T., Saito, S., Sapp, R. K., Murthy, K., et al. (2007). Production of infectious hepatitis C virus of various genotypes in cell cultures. Journal of Virology, 81, 4405–4411. doi:10.1128/JVI.02334-06.
Kato, T., & Wakita, T. (2005). Production of infectious hepatitis C virus in cell culture. Uirusu, 55, 287–295. doi:10.2222/jsv.55.287.
Li, X., Jeffers, L. J., Shao, L., Reddy, K. R., de Medina, M., Scheffel, J., et al. (1995). Identification of hepatitis C virus by immunoelectron microscopy. Journal of Viral Hepatitis, 2, 227–234. doi:10.1111/j.1365-2893.1995.tb00034.x.
Lin, L., Fevery, J., & Hiem Yap, S. (2002). A novel strand-specific RT-PCR for detection of hepatitis C virus negative-strand RNA (replicative intermediate): Evidence of absence or very low level of HCV replication in peripheral blood mononuclear cells. Journal of Virological Methods, 100, 97–105. doi:10.1016/S0166-0934(01)00399-8.
Lindenbach, B. D., Evans, M. J., Syder, A. J., Wolk, B., Tellinghuisen, T. L., Liu, C. C., et al. (2005). Complete replication of hepatitis C virus in cell culture. Science, 309, 623–626. doi:10.1126/science.1114016.
Lohmann, V., Korner, F., Koch, J., Herian, U., Theilmann, L., & Bartenschlager, R. (1999). Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science, 285, 110–113. doi:10.1126/science.285.5424.110.
Matsuo, E., Tani, H., Lim, C., Komoda, Y., Okamoto, T., Miyamoto, H., et al. (2006). Characterization of HCV-like particles produced in a human hepatoma cell line by a recombinant baculovirus. Biochemical and Biophysical Research Communications, 340, 200–208.
McCormick, C. J., Challinor, L., Macdonald, A., Rowlands, D. J., & Harris, M. (2004). Introduction of replication-competent hepatitis C virus transcripts using a tetracycline-regulable baculovirus delivery system. The Journal of General Virology, 85, 429–439. doi:10.1099/vir.0.19676-0.
McCormick, C. J., Rowlands, D. J., & Harris, M. (2002). Efficient delivery and regulable expression of hepatitis C virus full-length and minigenome constructs in hepatocyte-derived cell lines using baculovirus vectors. The Journal of General Virology, 83, 383–394.
Moradpour, D., Evans, M. J., Gosert, R., Yuan, Z., Blum, H. E., Goff, S. P., et al. (2004). Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. Journal of Virology, 78, 7400–7409. doi:10.1128/JVI.78.14.7400-7409.2004.
Murakami, K., Ishii, K., Ishihara, Y., Yoshizaki, S., Tanaka, K., Gotoh, Y., et al. (2006). Production of infectious hepatitis C virus particles in three-dimensional cultures of the cell line carrying the genome-length dicistronic viral RNA of genotype 1b. Virology, 351, 381–392. doi:10.1016/j.virol.2006.03.038.
Myung, J., Khalap, N., Kalkeri, G., Garry, R., & Dash, S. (2001). Inducible model to study negative strand RNA synthesis and assembly of hepatitis C virus from a full-length cDNA clone. Journal of Virological Methods, 94, 55–67. doi:10.1016/S0166-0934(01)00278-6.
O’Reilly, D. R., Miller, L. K., & Luckow, V. A. (1992). Baculovirus expression vectors: A Laboratory Manual. New York: Oxford University Press.
Ourlin, J. C., Vilarem, M. J., Daujat, M., Harricane, M. C., Domergue, J., Joyeux, H., et al. (1997). Lipid-mediated transfection of normal adult human hepatocytes in primary culture. Analytical Biochemistry, 247, 34–44. doi:10.1006/abio.1997.2025.
Pietschmann, T., Kaul, A., Koutsoudakis, G., Shavinskaya, A., Kallis, S., Steinmann, E., et al. (2006). Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proceedings of the National Academy of Sciences of the United States of America, 103, 7408–7413. doi:10.1073/pnas.0504877103.
Pietschmann, T., Lohmann, V., Kaul, A., Krieger, N., Rinck, G., Rutter, G., et al. (2002). Persistent and transient replication of full-length hepatitis C virus genomes in cell culture. Journal of Virology, 76, 4008–4021. doi:10.1128/JVI.76.8.4008-4021.2002.
Raty, J. K., Airenne, K. J., Marttila, A. T., Marjomaki, V., Hytonen, V. P., Lehtolainen, P., et al. (2004). Enhanced gene delivery by avidin-displaying baculovirus. Molecular Therapy, 9, 282–291. doi:10.1016/j.ymthe.2003.11.004.
Sandig, V., Hofmann, C., Steinert, S., Jennings, G., Schlag, P., & Strauss, M. (1996). Gene transfer into hepatocytes and human liver tissue by baculovirus vectors. Human Gene Therapy, 7, 1937–1945. doi:10.1089/hum.1996.7.16-1937.
Seeff, L. B. (2002). Natural history of chronic hepatitis C. Hepatology (Baltimore, Md.), 36, S35–S46. doi:10.1002/hep.1840360706.
Shimakami, T., Honda, M., Kusakawa, T., Murata, T., Shimotohno, K., Kaneko, S., et al. (2006). Effect of hepatitis C virus (HCV) NS5B-nucleolin interaction on HCV replication with HCV subgenomic replicon. Journal of Virology, 80, 3332–3340. doi:10.1128/JVI.80.7.3332-3340.2006.
Shoji, I., Aizaki, H., Tani, H., Ishii, K., Chiba, T., Saito, I., et al. (1997). Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. The Journal of General Virology, 78(Pt 10), 2657–2664.
Simmonds, P., Bukh, J., Combet, C., Deleage, G., Enomoto, N., Feinstone, S., et al. (2005). Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology (Baltimore, Md.), 42, 962–973. doi:10.1002/hep. 20819.
Song, J., Liang, C., & Chen, X. (2006). Transduction of avian cells with recombinant baculovirus. Journal of Virological Methods, 135, 157–162. doi:10.1016/j.jviromet.2006.02.013.
Triyatni, M., Vergalla, J., Davis, A. R., Hadlock, K. G., Foung, S. K., & Liang, T. J. (2002). Structural features of envelope proteins on hepatitis C virus-like particles as determined by anti-envelope monoclonal antibodies and CD81 binding. Virology, 298, 124–132. doi:10.1006/viro.2002.1463.
Wakita, T., Pietschmann, T., Kato, T., Date, T., Miyamoto, M., Zhao, Z., et al. (2005). Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nature Medicine, 11, 791–796. doi:10.1038/nm1268.
Yi, M., Villanueva, R. A., Thomas, D. L., Wakita, T., & Lemon, S. M. (2006). Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proceedings of the National Academy of Sciences of the United States of America, 103, 2310–2315. doi:10.1073/pnas.0510727103.
Zhong, J., Gastaminza, P., Cheng, G., Kapadia, S., Kato, T., Burton, D. R., et al. (2005). Robust hepatitis C virus infection in vitro. Proceedings of the National Academy of Sciences of the United States of America, 102, 9294–9299. doi:10.1073/pnas.0503596102.
Zolotukhin, S. (2005). Production of recombinant adeno-associated virus vectors. Human Gene Therapy, 16, 551–557. doi:10.1089/hum.2005.16.551.
Acknowledgments
We are grateful to Dr. Simon Rayner for a critical review of the manuscript. The authors are grateful to professor Ralf Bartenschlager (University of Heidelberg) for providing the Huh7-lunet cell line. The work was supported by National Basic Research Program (2005CB522901).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, X., Han, Q., Song, J. et al. Baculovirus Mediated Production of Infectious Hepatitis C Virus in Human Hepatoma Cells Stably Expressing T7 RNA Polymerase. Mol Biotechnol 40, 186–194 (2008). https://doi.org/10.1007/s12033-008-9075-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-008-9075-2