Abstract
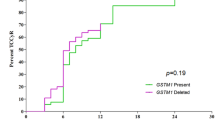
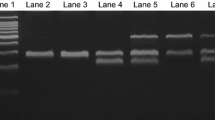
The glutathione S-transferases (GSTs) are phase II xenobiotic metabolizing enzymes known to be involved in the detoxification of carcinogens and anticancer drugs. Individual genetic variation linked to inherited polymorphisms of GSTT1 and GSTM1 leading to a complete loss of enzyme activity could expose subjects to develop cancer or to induce drug resistance. Indeed, despite the impressive results obtained with the imatinib, some patients with chronic myeloid leukemia (CML) fail to achieve the expected results or develop resistance. The present study aimed to examine the impact of GSTT1 and GSTM1 polymorphisms on the response to imatinib in patients with CML. Multiplex polymerase chain reaction was used to detect the genotypes of GSTT1 and GSTM1 in 60 CML patients. We found that side effects were more frequent in patients carrying GSTT1 null when compared to GSTT1 present carriers (31 vs. 16.6 %; χ 2 = 6.2; p = 0.013). The loss of hematologic response was statistically greater in patients carrying the combined genotype GSTT1 present/GSTM1 present (26.3 %) when compared to GSTT1 null/GSTM1 present (12.8 %), GSTT1 present/GSTM1 null (8.3 %) and GSTT1 null/GSTM1 null (0 %), (χ 2 = 18.85; p < 0.001). The complete cytogenetic response was higher in patients harboring the GSTT1 null/GSTM1 null (75 %) compared with GSTT1 null/GSTM1 present (55.6 %), GSTT1 present/GSTM1 null (50 %) and GSTT1 present/GSTM1 present (47.8). On the other hand, the frequency of none cytogenetic responders was more common in patients carrying GSTT1 present/GSTM1 present (34.8 %) when compared to other genotype combinations (χ 2 = 20.99; p = 0.05). Moreover, the GSTT1 present/GSTM1 present appeared to be associated with a final dose of 600 or 800 mg of imatinib, but not significantly. Based on these findings, we find that the interaction between GSTT1 and GSTM1 seems to influence treatment outcome in patients with CML. Therefore, further investigations are required to confirm these results, for better genotype–phenotype correlation.




Similar content being viewed by others
References
Deininger MWN, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–56.
Schiffer CA. BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia. N Engl J Med. 2007;357:258–65. http://www.ncbi.nlm.nih.gov/pubmed/17634461.
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. http://www.ncbi.nlm.nih.gov/pubmed/17151364.
Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, et al. Imatinib compared with interferon and low-dose cytarabine for newly-diagnosed chronic-phase chronicmyeloid leukemia. N Engl J Med. 2003;348:994–1004.
Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–83. http://www.ncbi.nlm.nih.gov/pubmed/12623848.
Werner B, Lutz D, Brümmendorf TH, Traulsen A, Balabanov S. Dynamics of resistance development to imatinib under increasing selection pressure: a combination of mathematical models and in vitro data. PLoS ONE. 2011;6:1–8.
Bose P, Park H, Al-Khafaji J, Grant S. Strategies to circumvent the T315I gatekeeper mutation in the Bcr-Abl tyrosine kinase. Leuk Res Rep. 2013;2:18–20.
Vivona D, Bueno CT, Lima LT, Hirata RDC, Hirata MH, Luchessi AD, et al. ABCB1 haplotype is associated with major molecular response in chronic myeloid leukemia patients treated with standard-dose of imatinib. Blood cells Mol Dis. 2012;48:132–6. http://www.ncbi.nlm.nih.gov/pubmed/22134106.
Kassogue Y, Quachouh M, Dehbi H, Quessar A, Benchekroun S, Nadifi S. Functional polymorphism of CYP2B6 G15631T is associated with hematologic and cytogenetic response in chronic myeloid leukemia patients treated with imatinib. Med Oncol. (Northwood, London, England). 2014;31:782–6. http://www.ncbi.nlm.nih.gov/pubmed/24293093.
Villuendas R, Steegmann JL, Pollán M, Tracey L, Granda a, Fernández-Ruiz E, et al. Identification of genes involved in imatinib resistance in CML: a gene-expression profiling approach. Leukemia. 2006;20:1047–54. http://www.ncbi.nlm.nih.gov/pubmed/16598311.
Dulucq S, Bouchet S, Turcq B, Lippert E, Etienne G, Reiffers J, et al. Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2008;112:2024–7. http://www.ncbi.nlm.nih.gov/pubmed/18524988.
Vivona D, Bueno CT, Lima LT, Hirata RDC, Hirata MH, Luchessi AD, et al. ABCB1 haplotype is associated with major molecular response in chronic myeloid leukemia patients treated with standard-dose of imatinib. Blood Cells Mol Dis. 2012;48:132–6. http://www.ncbi.nlm.nih.gov/pubmed/22134106.
Sailaja K, Surekha D, Rao DN, Rao DR, Vishnupriya S. Association of the GSTP1 gene (Ile105Val) polymorphism with chronic myeloid leukemia. Asian Pac J Cancer Prev. APJCP [Internet]. 2010;11:461–4. http://www.ncbi.nlm.nih.gov/pubmed/20843134.
Koh Y, Kim D-Y, Park S-H, Jung S-H, Park E, Kim H-J, et al. GSTT1 copy number gain is a poor predictive marker for escalated-dose imatinib treatment in chronic myeloid leukemia: genetic predictive marker found using array comparative genomic hybridization. Cancer Genet Cytogenet. 2010;203:215–21. http://www.ncbi.nlm.nih.gov/pubmed/21156236.
Elhoseiny S, El-wakil M, Fawzy M, Rahman AA. GSTP1 (Ile105Val) gene polymorphism: risk and treatment response in chronic myeloid leukemia. J Cancer Ther. 2014;5:1–10.
Boyer TD. The glutathione S-transferases: an update. Hepatology. 1989;9:486–96. http://www.ncbi.nlm.nih.gov/pubmed/2646197.
Mannervik B, Awasthi YC, Board PG, Hayes JD, Di llo C, Ketterer B, et al. Nomenclature for human glutathione transferases. Biochem J. 1992;282:305–6.
Pearson WR, Vorachek WR, Xu SJ, Berger R, Hart I, Vannais D, et al. Identification of class-mu glutathione transferase genes GSTM1 GSTM5 on human chromosome 1p13. Am J Hum Genet. 1993;53:220–33.
Webb G, Vaska V, Coggan M, Board P. Chromosomal localization of the gene for the human theta class glutathione transferase (GSTT1). Genomics. 1996;33:121–3. http://www.ncbi.nlm.nih.gov/pubmed/8617495.
Ruzza P, Calderan A. Glutathione transferase (GST)-activated prodrugs. Pharmaceutics. 2013;5:220–31.
O’Brien ML, Tew KD. Glutathione and related enzymes in multidrug resistance. Eur J Cancer (Oxford, England: 1990). 1996;32A:967–78. http://www.ncbi.nlm.nih.gov/pubmed/10500793.
Stanulla M, Schrappe M, Brechlin AM, Zimmermann M, Welte K, Mu A. Polymorphisms within glutathione S-transferase genes (GSTM1, GSTT1, GSTP1) and risk of relapse in childhood B-cell precursor acute lymphoblastic leukemia: a case-control study. Blood. 2000;95:1222–8.
Ambrosone CB, Sweeney C, Coles BF, Thompson PA, Mcclure GY, Korourian S, et al. Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer after treatment for breast cancer 1. Cancer Res. 2001;61:7130–5.
Chen CL, Liu Q, Pui CH, Rivera GK, Sandlund JT, Ribeiro R, et al. Higher frequency of glutathione S-transferase deletions in black children with acute lymphoblastic leukemia. Blood. 1997;89:1701–7. http://www.ncbi.nlm.nih.gov/pubmed/9057653.
Davies S, Bhatia S, Ross J, Kiffmeyer W, Gaynon P, Radloff G, et al. Glutathione S-transferase P1 genotypes, genetic susceptibility and outcome of therapy in thai childhood acute lymphoblastic leukemia. Blood [Internet]. 2002;100:67–71. http://www.ncbi.nlm.nih.gov/pubmed/17696749.
Deininger MW, O’Brien SG, Ford JM, Druker BJ. Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol. 2003;21:1637–47. http://www.ncbi.nlm.nih.gov/pubmed/12668652.
Kantarjian HM, O’Brien S, Cortes J, Giles FJ, Rios MB, Shan J, et al. Imatinib mesylate therapy improves survival in patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in the chronic phase: comparison with historic data. Cancer. 2003;98:2636–42. http://www.ncbi.nlm.nih.gov/pubmed/14669283.
Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Voso MT, D’Alo’ F, Putzulu R, Mele L, Scardocci A, Chiusolo P, et al. Negative prognostic value of glutathione S-transferase (GSTM1 and GSTT1) deletions in adult acute myeloid leukemia. Blood. 2002;100:2703–7. http://www.ncbi.nlm.nih.gov/pubmed/12351375.
Smith CM, Kelsey KT, Wiencke JK, Leyden K, Levin S, Christiani DC. Inherited glutathione-S-transferase deficiency is a risk factor for pulmonary asbestosis. Cancer Epidemiol Biomarkers Prev. 1994;3:471–7. http://www.ncbi.nlm.nih.gov/pubmed/8000297.
McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–48. rhttp://www.ncbi.nlm.nih.gov/pubmed/16550164.
Mossallam GI, Abdel Hamid TM, Samra M a. Glutathione S-transferase GSTM1 and GSTT1 polymorphisms in adult acute myeloid leukemia; its impact on toxicity and response to chemotherapy. J Egypt Natl Cancer Inst. 2006;18:264–73. http://www.ncbi.nlm.nih.gov/pubmed/17671537.
Acknowledgments
The authors thank the Hassan II Academy of Science and Technology for the financial support.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kassogue, Y., Quachouh, M., Dehbi, H. et al. Effect of interaction of glutathione S-transferases (T1 and M1) on the hematologic and cytogenetic responses in chronic myeloid leukemia patients treated with imatinib. Med Oncol 31, 47 (2014). https://doi.org/10.1007/s12032-014-0047-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0047-z