Abstract
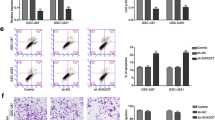
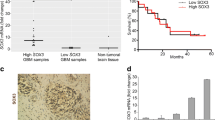
SOX9 belongs to the SOX (Sry-related high-mobility group box) family and acts as a transcription factor that plays a central role in the development and differentiation of multiple cell lineages. Recent studies have demonstrated that SOX9 is required for the carcinogenesis in several cancer types. The aim of this study was to investigate the clinicopathological significance of SOX9 expression in human malignant glioma. SOX9 mRNA expression was detected by real-time quantitative RT-PCR assay in glioma and nonneoplastic brain tissues. Then, the association of SOX9 mRNA expression with clinicopathological factors or prognosis of glioma patients was statistically analyzed. In addition, the small interfering RNA was used to knockdown SOX9 expression in a glioma cell line and to analyze the effects of SOX9 inhibition on cell growth, cell cycle and apoptosis of glioma cell line. The expression level of SOX9 mRNA in glioma tissues was significantly higher than that in corresponding nonneoplastic brain tissues (P < 0.001). In addition, a high level of SOX9 mRNA expression was significantly more common in glioma tissues with advanced WHO grade than those with low grade (P = 0.02). The increased expression of SOX9 mRNA was also significantly correlated with low Karnofsky performance score (P = 0.008). Meanwhile, the disease-free and overall survival rates of patients with high SOX9 mRNA expression were obviously lower than those of patients with low SOX9 mRNA expression (both P = 0.01). Multivariate analysis showed that high SOX9 mRNA expression was an independent prognostic factor for glioma patients (P = 0.02). Moreover, the down-regulation of SOX9 could inhibit the cell growth, induce the cell arrest in G2/M phase of cell cycle and enhance the apoptosis in glioma cells. Our data suggest for the first time that the over-expression of SOX9 mRNA is closely associated with poor clinical outcome of patients with malignant gliomas, and targeting SOX9 may be a novel therapeutic strategy for this tumor.



Similar content being viewed by others
References
Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93.
He SM, Zhao ZW, Wang Y, et al. Potential role of Jun activation domain-binding protein 1 and phosphorylated p27 expression in prognosis of glioma. Brain Tumor Pathol. 2012;29:3–9.
Curran WJ Jr, Scott CB. Radiosurgery for glioma patients: hope or hype? Int J Radiat Oncol Biol Phys. 1996;36:1279–80.
Hersmus R, Kalfa N, de Leeuw B, et al. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD). J Pathol. 2008;215:31–8.
Jay P, Berta P, Blache P. Expression of the carcinoembryonic antigen gene is inhibited by SOX9 in human colon carcinoma cells. Cancer Res. 2005;65:2193–8.
Thomsen MK, Ambroisine L, Wynn S, et al. SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation. Cancer Res. 2010;70:979–87.
Sashikawa Kimura M, Mutoh H, Sugano K. SOX9 is expressed in normal stomach, intestinal metaplasia, and gastric carcinoma in humans. J Gastroenterol. 2011;46:1292–9.
Chakravarty G, Moroz K, Makridakis NM, et al. Prognostic significance of cytoplasmic SOX9 in invasive ductal carcinoma and metastatic breast cancer. Exp Biol Med (Maywood). 2011;236:145–55.
Sutter R, Shakhova O, Bhagat H, et al. Cerebellar stem cells act as medulloblastoma-initiating cells in a mouse model and a neural stem cell signature characterizes a subset of human medulloblastomas. Oncogene. 2010;29:1845–56.
Schlierf B, Friedrich RP, Roerig P, Felsberg J, Reifenberger G, Wegner M. Expression of SoxE and SoxD genes in human gliomas. Neuropathol Appl Neurobiol. 2007;33:621–30.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classifcation of tumors of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Hanley KP, Oakley F, Sugden S, Wilson DI, Mann DA, Hanley NA. Ectopic SOX9 mediates extracellular matrix deposition characteristic of organ fibrosis. J Biol Chem. 2008;283:14063–71.
Malki S, Bibeau F, Notarnicola C, et al. Expression and biological role of the prostaglandin D synthase/SOX9 pathway in human ovarian cancer cells. Cancer Lett. 2007;255:182–93.
Gasca S, Canizares J, De Santa Barbara P, et al. A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination. Proc Natl Acad Sci USA. 2002;99:11199–204.
Wang H, McKnight NC, Zhang T, Lu ML, Balk SP, Yuan X. SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res. 2007;67:528–36.
Lü B, Fang Y, Xu J, et al. Analysis of SOX9 expression in colorectal cancer. Am J Clin Pathol. 2008;130:897–904.
Aleman A, Adrien L, Lopez-Serra L, et al. Identification of DNA hypermethylation of SOX9 in association with bladder cancer progression using CpG microarrays. Br J Cancer. 2008;98:466–73.
Chakravarty G, Rider B, Mondal D. Cytoplasmic compartmentalization of SOX9 abrogates the growth arrest response of breast cancer cells that can be rescued by trichostatin A treatment. Cancer Biol Ther. 2011;11:71–83.
Passeron T, Valencia JC, Namiki T, et al. Upregulation of SOX9 inhibits the growth of human and mouse melanomas and restores their sensitivity to retinoic acid. J Clin Invest. 2009;119:954–63.
Vidal VP, Ortonne N, Schedl A. SOX9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J Cutan Pathol. 2008;35:373–9.
Wang H, Leav I, Ibaragi S, et al. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 2008;68:1625–30.
Jiang SS, Fang WT, Hou YH, et al. Upregulation of SOX9 in lung adenocarcinoma and its involvement in the regulation of cell growth and tumorigenicity. Clin Cancer Res. 2010;16:4363–73.
Acknowledgments
This work was funded by National Natural Science Foundation of China (NO. 81101736) and Talents Supported Plan Foundation of Tangdu Hospital (2011)
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Liang Wang, Shiming He, and Jun Yuan contributed equally.
Rights and permissions
About this article
Cite this article
Wang, L., He, S., Yuan, J. et al. Oncogenic role of SOX9 expression in human malignant glioma. Med Oncol 29, 3484–3490 (2012). https://doi.org/10.1007/s12032-012-0267-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0267-z