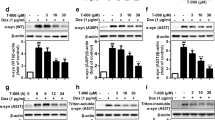
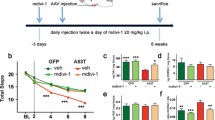
Abstract
Bcl-2-associated athanogene-1 (BAG1) is a multifunctional protein comprising co-chaperone function, increasing Hsp70 foldase activity and chaperone-dependent protein degradation of misfolded substrates, with anti-apoptotic activity. It is neuroprotective in different models of neurological diseases, like cerebral ischemia and Huntington’s disease. In the context of Parkinson’s disease, it has recently been shown to restore DJ-1 function in an in vitro model of hereditary Parkinson’s disease. Here, we demonstrate that BAG1 overexpression in SH-SY5Y cells reduces toxicity after transfection of disease-related α-synuclein mutants. Furthermore, it protects from rotenone-induced cell death in vitro and ameliorates neuronal demise in an in vivo 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine (MPTP) model for Parkinson’s disease after adeno-associated virus (AAV)-mediated BAG1 gene transfer into the substantia nigra in mice but showed no protective effects in an in vitro 6-hydroxidopamine model. In conclusion, we present BAG1 as a potential therapeutic target in Parkinson’s disease.





Similar content being viewed by others
References
Anderson DW, Bradbury KA, Schneider JS (2006) Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci 24:3174–3182
Blum D, Torch S, Lambeng N et al (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:135–172
Bove J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2:484–494
Burke D, Gasdaska P, Hartwell L (1989) Dominant effects of tubulin overexpression in Saccharomyces cerevisiae. Mol Cell Biol 9:1049–1059
Cook C, Stetler C, Petrucelli L (2012) Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb Perspect Med 2:a009423
de Rijk MC, Launer LJ, Berger K et al (2000) Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurol Dis Elder Res Group Neurol 54:S21–S23
Deeg S, Gralle M, Sroka K, Bahr M, Wouters FS, Kermer P (2010) BAG1 restores formation of functional DJ-1 L166P dimers and DJ-1 chaperone activity. J Cell Biol 188:505–513
Demand J, Alberti S, Patterson C, Hohfeld J (2001) Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr Biol 11:1569–1577
Dohm CP, Kermer P, Bahr M (2008) Aggregopathy in neurodegenerative diseases: mechanisms and therapeutic implication. Neurodegener Dis 5:321–338
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 28:889–901
Glinka Y, Tipton KF, Youdim MB (1996) Nature of inhibition of mitochondrial respiratory complex I by 6-Hydroxydopamine. J Neurochem 66:2004–2010
Goldberg MS, Lansbury PT Jr (2000) Is there a cause-and-effect relationship between alpha-synuclein fibrillization and Parkinson’s disease? Nat Cell Biol 2:E115–E119
Hanrott K, Gudmunsen L, O'Neill MJ, Wonnacott S (2006) 6-hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cdelta. J Biol Chem 281:5373–5382
Jiang H, Jiang Q, Feng J (2004) Parkin increases dopamine uptake by enhancing the cell surface expression of dopamine transporter. J Biol Chem 279:54380–54386
Kermer P, Krajewska M, Zapata JM et al (2002) Bag1 is a regulator and marker of neuronal differentiation. Cell Death Differ 9:405–413
Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ (2004) Hsp70 reduces alpha-synuclein aggregation and toxicity. J Biol Chem 279:25497–25502
Liman J, Ganesan S, Dohm CP et al (2005) Interaction of BAG1 and Hsp70 mediates neuroprotectivity and increases chaperone activity. Mol Cell Biol 25:3715–3725
Lotharius J, Brundin P (2002) Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum Mol Genet 11:2395–2407
Luders J, Demand J, Hohfeld J (2000) The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem 275:4613–4617
Mochizuki H, Hayakawa H, Migita M et al (2001) An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson’s disease. Proc Natl Acad Sci U S A 98:10918–10923
Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36:2503–2508
Offen D, Beart PM, Cheung NS et al (1998) Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine neurotoxicity. Proc Natl Acad Sci U S A 95:5789–5794
Opazo F, Krenz A, Heermann S, Schulz JB, Falkenburger BH (2008) Accumulation and clearance of alpha-synuclein aggregates demonstrated by time-lapse imaging. J Neurochem 106:529–540
Outeiro TF, Putcha P, Tetzlaff JE et al (2008) Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS One 3:e1867
Paterna JC, Feldon J, Bueler H (2004) Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol 78:6808–6817
Perumal AS, Gopal VB, Tordzro WK, Cooper TB, Cadet JL (1992) Vitamin E attenuates the toxic effects of 6-hydroxydopamine on free radical scavenging systems in rat brain. Brain Res Bull 29:699–701
Planchamp V, Bermel C, Tonges L et al (2008) BAG1 promotes axonal outgrowth and regeneration in vivo via Raf-1 and reduction of ROCK activity. Brain 131:2606–2619
Przedborski S, Vila M (2003) The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson’s disease. Ann. N.Y. Acad Sci 991:189–198
Schoch S, Cibelli G, Thiel G (1996) Neuron-specific gene expression of synapsin I. Major role of a negative regulatory mechanism. J Biol Chem 271:3317–3323
Schuler F, Casida JE (2001) Functional coupling of PSST and ND1 subunits in NADH:ubiquinone oxidoreductase established by photoaffinity labeling. Biochim Biophys Acta 1506:79–87
Shin Y, Klucken J, Patterson C, Hyman BT, McLean PJ (2005) The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysosomal pathways. J Biol Chem 280:23727–23734
Sroka K, Voigt A, Deeg S et al (2009) BAG1 modulates huntingtin toxicity, aggregation, degradation, and subcellular distribution. J Neurochem 111:801–807
Storch A, Kaftan A, Burkhardt K, Schwarz J (2000) 6-Hydroxydopamine toxicity towards human SH-SY5Y dopaminergic neuroblastoma cells: independent of mitochondrial energy metabolism. J Neural Transm 107:281–293
Takayama S, Reed JC (2001) Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol 3:E237–E241
Takayama S, Sato T, Krajewski S et al (1995) Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell 80:279–284
Takayama S, Krajewski S, Krajewska M et al (1998) Expression and location of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissues and tumor cell lines. Cancer Res 58:3116–3131
Tatton NA, Kish SJ (1997) In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 77:1037–1048
Vila M, Jackson-Lewis V, Vukosavic S et al (2001) Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A 98:2837–2842
Wu Y, Blum D, Nissou MF, Benabid AL, Verna JM (1996) Unlike MPP+, apoptosis induced by 6-OHDA in PC12 cells is independent of mitochondrial inhibition. Neurosci Lett 221:69–71
Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L (2009) Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One 4:e5515
Yang L, Matthews RT, Schulz JB et al (1998) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J Neurosci 18:8145–8152
Funding Source
The work was supported by the DFG Research Center for Molecular Physiology of the Brain (CMPB), Göttingen, Germany, the NeuroNE network of excellence within the 6th framework program of the European Union, and Starter Grants of the University Medical Center Goettingen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pawel Kermer and Anja Köhn contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Stable expression in SH-SY5Y cells was shown via western blotting using a BAG1 antibody. Expression of BAG1 can only be seen in cells stably overexpressing BAG1, but not in the empty vector transfected cells. β-Tubulin staining served as protein loading control. (JPEG 7 kb)
Supplementary Fig. S2
Expression of the different α-Synuclein constructs on protein level was tested via western blotting against α-Synuclein. The expression levels of the different mutants are comparable. β-Tubulin staining served as protein loading control. (JPEG 26 kb)
Supplementary Fig. S3
Expression of the BAG1 protein after viral transduction of the SN was tested via immunohistochemistry with a) staining against BAG1 b) viral EGFP expression. As shown in the merged picture EGFP and BAG1 are co localising (c). (scale bar = 50 μm) (JPEG 26 kb)
Rights and permissions
About this article
Cite this article
Kermer, P., Köhn, A., Schnieder, M. et al. BAG1 is Neuroprotective in In Vivo and In Vitro Models of Parkinson’s Disease. J Mol Neurosci 55, 587–595 (2015). https://doi.org/10.1007/s12031-014-0396-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0396-2