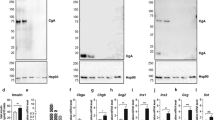
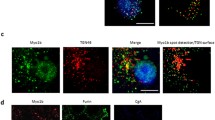
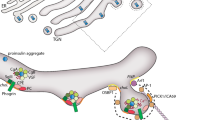
Abstract
Biogenesis and post-Golgi transport of peptidergic secretory granules to the release site are crucial for secretion of neuropeptides from neuroendocrine cells. Recent studies have uncovered multilevel molecular mechanisms for the regulation of secretory granule biogenesis. Insulinoma-associated protein 2 (ICA512/IA-2), polypyrimidine-tract binding protein, and chromogranin A have been identified to regulate secretory granule biogenesis at the transcriptional, posttranscriptional, and posttranslational levels, respectively, by increasing granule protein levels, which in turn drives granule formation after stimulation. Post-Golgi transport of secretory granules is microtubule-based and mediated by transmembrane carboxypeptidase E (CPE). The cytoplasmic tail of CPE anchors secretory granules to the microtubule motors, kinesin-2 and -3, or dynein, via interaction with the adaptor, dynactin, to mediate anterograde and retrograde transport, respectively.




Similar content being viewed by others
References
Alexander, K., Nikodemova, M., Kucerova, J., & Strbak, V. (2005). Colchicine treatment differently affects releasable thyrotropin-releasing hormone (TRH) pools in the hypothalamic paraventricular nucleus (PVN) and the median eminence (ME). Cellular and Molecular Neurobiology, 25, 681–695, doi:10.1007/s10571-005-4008-0.
Auweter, S. D., & Allain, F. H. (2007). Structure–function relationships of the polypyrimidine tract binding protein. Cellular and Molecular Life Science, 65, 516–527.
Bennett, H. S. (1941). Cytological manifestations of secretion in the adrenal medulla of the cat. The American Journal of Anatomy, 69, 333–381. doi:10.1002/aja.1000690302.
Beuret, N., Stettler, H., Renold, A., Rutishauser, J., & Spiess, M. (2004). Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. The Journal of Biological Chemistry, 279, 20242–20249, doi:10.1074/jbc.M310613200.
Berezuk, M. A., & Schroer, T. A. (2007). Dynactin enhances the processivity of kinesin-2. Traffic, 8, 124–129, doi:10.1111/j.1600-0854.2006.00517.x.
Blaschko, H., Comline, R. S., Schneider, F. H., Silver, M., & Smith, A. D. (1967). Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature, 215, 58–59, doi:10.1038/215058a0.
Bruce, A. W., Donaldson, I. J., Wood, I. C., Yerbury, S. A., Sadowski, M. I., Chapman, M., et al. (2004). Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proceedings of National Academy of Science U S A, 101, 10458–10463, doi:10.1073/pnas.0401827101.
Bruce, A. W., KreJci, A., Ooi, L., Wood, I. C., Dolezal, V., & Buckley, N. J. (2006). The transcriptional repressor REST is a critical regulator of the neurosecretory phenotype. Journal of Neurochemistry, 98, 1828–1840, doi:10.1111/j.1471-4159.2006.04010.x.
Calderone, A., Jover, T., Noh, K. M., Tanaka, H., Yokota, H., Lin, Y., et al. (2003). Ischemic insults derepress the gene silencer REST in neurons destined to die. The Journal of Neuroscience, 23, 2112–2121.
Chong, J. A., Tapia-Ramirez, J., Kim, S., Toledo-Aral, J. J., Zheng, Y., Boutros, M. C., et al. (1995). RESY: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell, 80, 949–957, doi:10.1016/0092-8674(95)90298-8.
Cool, D. R., Normant, E., Shen, F., Chen, H. C., Pannell, L., Zhang, Y., & Loh, Y. P. (1997). Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell, 88, 73–83, doi:10.1016/S0092-8674(00)81860-7.
Eaton, B. A., Haugwitz, M., Lau, D., & Moore, H. P. (2000). Biogenesis of regulated exocytotic carriers in neuroendocrine cells. Journal of Neuroscience, 20, 7334–7344.
Eiden, L. E., Giraud, P., Dave, J. R., Hotchkiss, A. J., & Affolter, H. U. (1984). Nicotinic receptor stimulation activates enkephalin release and biosynthesis in adrenal chromaffin cells. Nature, 312, 661–663, doi:10.1038/312661a0.
Dikeakos, J. D., & Reudelhuber, T. L. (2007). Sending proteins to dense core secretory granules: still a lot to sort out. The Journal of Cell Biology, 177, 191–196, doi:10.1083/jcb.200701024.
Gauthier, L. R., Charrin, B. C., Borrell-Pages, M., Dompierre, J. P., Rangone, H., Cordelieres, F. P., et al. (2004). Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell, 118, 127–138, doi:10.1016/j.cell.2004.06.018.
Gill, S. R., Schroer, T. A., Szilak, I., Steuer, E. R., Sheetz, M. P., & Cleveland, D. W. (1991). Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. Journal of Cell Biology, 115, 1639–1650, doi:10.1083/jcb.115.6.1639.
Grundschober, C., Malosio, M. L., Astolfi, L., Giordano, T., Nef, P., & Meldolesi, J. (2002). Neurosecretion competence. A comprehensive gene expression program identified in PC12 cells. Journal of Biological Chemistry, 277, 36715–36724 Erratum in: (2002). Journal of Biological Chemistry, 277, 46840. doi:10.1074/jbc.M203777200.
Hamm-Alvarez, S. F., Da Costa, S., Yang, T., Wei, X., Gierow, J. P., & Mircheff, A. K. (1997). Cholinergic stimulation of lacrimal acinar cells promotes redistribution of membrane-associated kinesin and the secretory protein, beta-hexosaminidase, and increases kinesin motor activity. Experimental Eye Research, 64, 141–156, doi:10.1006/exer.1996.0198.
Harashima, S., Clark, A., Christie, M. R., & Notkins, A. L. (2005). The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proceedings of National Academy of Science U S A, 102, 8704–8709, doi:10.1073/pnas.0408887102.
Hiremagalur, B., Nankova, B., Nitahara, J., Zeman, R., & Sabban, E. L. (1993). Nicotine increases expression of tyrosine hydroxylase gene. Involvement of protein kinase A-mediated pathway. The Journal of Biological Chemistry, 268, 23704–23711.
Huh, Y. H., Jeon, S. H., & Yoo, S. H. (2003). Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin. Journal of Biological Chemistry, 278, 40581–40589. doi:10.1074/jbc.M304942200.
Hendy, G. N., Li, T., Girard, M., Feldstein, R. C., Mulay, S., Desjardins, R., et al. (2006). Targeted ablation of the chromogranin a (Chga) gene: normal neuroendocrine dense-core secretory granules and increased expression of other granins. Molecular Endocrinology, 20, 1935–1947, doi:10.1210/me.2005-0398.
Hosaka, M., Watanabe, T., Sakai, Y., Uchiyama, Y., & Takeuchi, T. (2002). Identification of a chromogranin A domain that mediates binding to secretogranin III and targeting to secretory granules in pituitary cells and pancreatic beta-cells. Molecular Biology of Cell, 13, 3388–3399, doi:10.1091/mbc.02-03-0040.
Jacob, T. C., & Kaplan, J. M. (2003). The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. Journal of Neuroscience, 23, 2122–2130.
Kalinina, E., Varlamov, O., & Fricker, L. D. (2002). Analysis of the carboxypeptidase D cytoplasmic domain: Implications in intracellular trafficking. Journal of Cellular Biochemistry, 85, 101–111, doi:10.1002/jcb.10112.
Kilbourne, E. J., Nankova, B. B., Lewis, E. J., McMahon, A., Osaka, H., Sabban, D. B., et al. (1992). Regulated expression of the tyrosine hydroxylase gene by membrane depolarization. Identification of the responsive element and possible second messengers. The Journal of Biological Chemistry, 267, 7563–7569.
Kim, T., Gondre-Lewis, M. C., Arnauotova, I., & Loh, Y. P. (2006). Dense-core secretory granule biogenesis. Physiology (Bethesda), 21, 24–33. doi:10.1152/physiol.00043.2005.
Kim, T., & Loh, Y. P. (2006). Protein nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation. Molecular Biology of the Cell, 2, 789–798.
Kim, T., Tao-Cheng, J. H., Eiden, L. E., & Loh, Y. P. (2001). Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell, 106, 499–509, doi:10.1016/S0092-8674(01)00459-7.
Knoch, K. P., Bergert, H., Borgonovo, B., Saeger, H. D., Altkruger, A., Verkade, P., et al. (2004). Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nature Cell Biology, 6, 207–214, doi:10.1038/ncb1099.
Kondo, S., Sato-Yoshitake, R., Noda, Y., Aizawa, H., Nakata, T., Matsuura, Y., et al. (1994). KIF3A is a new microtubule-based anterograde motor in the nerve axon. Journal of Cell Biology, 125, 1095–1107, doi:10.1083/jcb.125.5.1095.
Loh, Y. P., Maldonado, A., Zhang, C., Tam, W. H., & Cawley, N. (2002). Mechanism of sorting proopiomelanocortin and proenkaphalin to the regulated secretory pathway of neuroendocrine cells. ANNALS of the New York Academy of Sciences, 971, 416–425.
Mahapatra, N. R., Mahata, M., O’Connor, D. T., & Mahata, S. K. (2003). Secretin activation of chromogranin A gene transcription. Identification of the signaling pathways in cis and in trans. The Journal of Biological Chemistry, 278, 19986–19994, doi:10.1074/jbc.M207983200.
Mahapatra, N. R., O’Connor, D. T., Vaingankar, S. M., HiKim, A. P., Mahata, M., Ray, S., et al. (2005). Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. The Journal of Clinical Investigation, 115, 1942–1952, doi:10.1172/JCI24354.
Malosio, M. L., Giordano, T., Laslop, A., & Meldolesi, J. (2004). Dense-core granules: a specific hallmark of the neuronal/neurosecretory cell phenotype. Journal of Cell Science, 117, 743–749, doi:10.1242/jcs.00934.
Palm, K., Belluardo, N., Metsis, M., & Timmusk, T. (1998). Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. Journal of Neuroscience, 18, 1280–1296.
Pance, A., Livesey, F. J., & Jackson, A. P. (2006). A role for the transcriptional repressor REST in maintaining the phenotype of neurosecretory-deficient PC12 cells. Journal of Neurochemistry, 99, 1435–1444, doi:10.1111/j.1471-4159.2006.04190.x.
Park, J. J., Cawley, N. X., & Loh, Y. P. (2008). Carboxypeptidase E cytoplasmic tail-driven vesicle transport is key for activity-dependent secretion of peptide hormones. Molecular Endocrinology, 22, 989–1005.
Rausch, D. M., Iacangelo, A. L., & Eiden, L. E. (1988). Glucocorticoid-and nerve growth factor-induced changes in chromogranin A expression define two different neuronal phenotypes in PC12 cells. Molecular Endocrinology, 2, 921–927.
Rudolf, R., Salm, T., Rustom, A., & Gerdes, H. H. (2001). Dynamics of immature secretory granules: role of cytoskeletal elements during transport, cortical restriction, and F-actin-dependent tethering. Molecular Biology of Cell, 12, 1353–1365.
Schoenherr, C. J., & Anderson, D. J. (1995). Silencing is golden: negative regulation in the control of neuronal gene transcription. Current Opinion in Neurobiology, 5, 566–571, doi:10.1016/0959-4388(95)80060-3.
Senda, T., & Yu, W. (1999). Kinesin cross-bridges between neurosecretory granules and microtubules in the mouse neurohypophysis. Neuroscience Letter, 262, 69–71, doi:10.1016/S0304-3940(99)00042-7.
Shakiryanova, D., Tully, A., & Levitan, E. S. (2006). Activity-dependent synaptic capture of transiting peptidergic vesicles. Nature Neuroscience, 9, 896–900, doi:10.1038/nn1719.
Tang, K., Wu, H., Mahata, S. K., Taupenot, L., Rozansky, D. J., Parmer, R. J., et al. (1996). Stimulus-transcription coupling in pheochromocytoma cells. Promoter region-specific activation of chromogranin a biosynthesis. The Journal of Biological Chemistry, 271, 28382–28390, doi:10.1074/jbc.271.45.28382.
Trajkovski, M., Mziaut, H., Altkruger, A., Ouwendijk, J., Knoch, K. P., Muller, S., et al. (2004). Nuclear translocation of an ICA512 cytosolic fragment couples granule exocytosis and insulin expression in beta-cells. The Journal of Cell Biology, 167, 1063–1074, doi:10.1083/jcb.200408172.
Varadi, A., Tsuboi, T., Johnson-Cadwell, L. I., Allan, V. J., & Rutter, G. A. (2003). Kinesin I and cytoplasmic dynein orchestrate glucose-stimulated insulin-containing vesicle movements in clonal MIN6 beta-cells. Biochemical and Biophysical Research Communications, 311, 272–282, doi:10.1016/j.bbrc.2003.09.208.
Varlamov, O., Kalinina, E., Chen, F. Y., & Fricker, L. D. (2001). Protein phosphatase 2A binds to the cytoplasmic tail of carboxypeptidase D and regulates post-trans-Golgi network trafficking. Journal of Cell Science, 114, 311–322.
Yamazaki, H., Nakata, T., Okada, Y., & Hirokawa, N. (1995). KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. Journal of Cell Biology, 130, 1387–1399, doi:10.1083/jcb.130.6.1387.
Yamazaki, H., Nakata, T., Okada, Y., & Hirokawa, N. (1996). Cloning and characterization of KAP3: a novel kinesin superfamily-associated protein of KIF3A/3B. Proceedings of National Academy of Science U S A, 93, 8443–8448, doi:10.1073/pnas.93.16.8443.
Acknowledgements
This research is supported by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.J., Koshimizu, H. & Loh, Y.P. Biogenesis and Transport of Secretory Granules to Release Site in Neuroendocrine Cells. J Mol Neurosci 37, 151–159 (2009). https://doi.org/10.1007/s12031-008-9098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9098-y