Abstract
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a largely reversible disease with long-term favorable outcome. A minority of patients, however, may develop progressive cerebral edema and ischemia resulting in severe disability or death. We report a case of severe intracranial hypertension associated with PRES that was successfully treated according to intracranial pressure (ICP)- and cerebral perfusion pressure (CPP)-driven therapy.
Methods
Case report.
Results
A 42-year-old woman underwent bilateral lung transplantation for severe bronchiectasis. Her immunosuppressive regimen consisted of azathioprine, prednisone, and tacrolimus. She acutely developed an aggressive form of PRES that rapidly resulted in severe refractory intracranial hypertension despite discontinuation of potentially causative medications and adequate supportive therapy. Accordingly, second-tier therapies, including barbiturate infusion, were instituted and immunosuppression was switched to anti-thymocyte globulin followed by mycophenolate mofetil. Within 10 h of barbiturate administration, ICP dropped to 20 mmHg. Thiopental was administered for two days and then rapidly tapered because of severe urosepsis. Six months after discharge from the intensive care unit the patient returned to near-normal life, her only complaint being short-term amnesia.
Conclusions
The decision to undertake ICP monitoring in medical conditions in which no clear recommendations exist greatly relies on physicians’ judgment. This case suggests that ICP monitoring may be considered in the setting of acute PRES among selected patients, when severe intracranial hypertension is suspected, provided that a multidisciplinary team of neurocritical care specialists is readily available.
Similar content being viewed by others
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a rare but potentially serious neurologic disorder mainly associated with hypertension, renal failure, immunologic diseases, and treatment with immunosuppressive drugs, like the calcineurin inhibitors. The pathophysiology of PRES is controversial, but clinical and experimental data indicate two possible mechanisms: endothelial damage potentially leading to uninhibited platelet aggregation and enhanced vasoconstriction, and/or hypertension-induced impairment of the autoregulatory mechanisms, especially in the vessels arising from the basilar artery, relatively devoid of sympathetic innervation [1, 2]. Immunosuppressive drugs are thought to cause PRES mainly through endothelial cell activation with the release of multiple mediators and pro-inflammatory cytokines, endothelin and nitric oxide depletion. All these mechanisms may result in altered vascular tone and in blood–brain barrier dysfunction. However, regardless of the etiology, the principal pathological feature in PRES is brain vasogenic edema, the pathogenesis of which is likely related to altered vascular tone and increased vascular permeability, followed by leakage of fluid into the brain interstitium [2, 3] (Table 1).
PRES usually has a benign course, when a prompt diagnosis is obtained and supportive therapy, in addition to withdrawal of culprit drugs, is warranted [1, 4]. However, patients may still develop progressive cerebral edema and ischemia resulting in severe disability or even death [5, 6]. If uncontrolled, vasogenic edema may progress into cytotoxic edema, ischemia and infarction. While ischemia is an irreversible process, brain edema and intracranial hypertension are potentially treatable complications of PRES, which should prompt aggressive treatment [5]. As there is no specific therapy, treatment mainly relies on supportive measures and withdrawal of all potentially causative factors. Thorough search for the precipitating drugs and/or conditions appears therefore mandatory.
Materials and Methods
We reviewed the clinical records of a single lung-transplant patient. Values of mean arterial pressure (MAP) and ICP were continuously recorded through an analog–digital converter (MacLab; ADInstruments Pty Ltd., Castle Hill, Australia) and CPP calculated as MAP–ICP.
Results
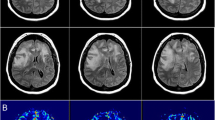
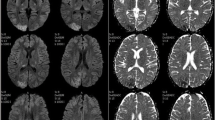
A 42-year-old woman underwent double-lung transplantation for severe bronchiectasis. Her post-transplant immunosuppressive regimen consisted of prednisone, azathioprine, and tacrolimus. Weaning from mechanical ventilation proved difficult because of respiratory failure due to primary graft dysfunction. On postoperative day 14, she presented a transient hypertensive crisis and sudden headache, immediately followed by unresponsiveness and gaze deviation. The patient was afebrile, cerebrospinal fluid analysis, including opening pressure, was unremarkable, and a non-contrast computed tomography was normal. The electroencephalogram, however, revealed continuous non-convulsive seizures consistent with complex partial status epilepticus in the left frontal and right central temporal regions. Supportive therapy with heavy sedation and intravenous phenytoin load (15 mg/kg) followed by 100 mg IV q8 h was undertaken. Magnetic resonance imaging of the brain showed bilateral hyperintense subcortical lesions in the temporo-parieto-occipital lobes, the corpus callosum, both thalami, hippocampi, and amygdalae in T2-weighted and fluid-attenuated inversion recovery images (Fig. 1a, b). In diffusion-weighted imaging sequences (Fig. 1c, d) the same regions showed slight restriction of mean diffusivity with increase of apparent diffusion coefficient values (Fig. 1e, f) suggestive of vasogenic edema. As the radiological and clinical picture was highly suspicious of PRES, tacrolimus was immediately discontinued. Noticeably, immunosuppressants could not be withdrawn or tapered at this stage after transplantation, and, therefore, it was decided to switch to cyclosporine, whereas azathioprine was continued. The next day sedation was interrupted, and the patient regained consciousness with no neurological deficits. However, the day after (postoperative day 16) she experienced generalized tonic–clonic seizures, which ceased rapidly after administration of IV diazepam. Oral levetiracetam (2,000 mg/day) was added to the former antiepileptic regimen. The patient regained consciousness, but remained drowsy. On day 21, the patient’s neurologic state suddenly worsened again, with rapid loss of consciousness, right > left anisocoria, III, VI, and VII right cranial nerve palsies. A contrast computed tomography scan showed diffuse brain swelling with hypodense lesions in the occipital and frontal lobes, and small, unspecific hypodensities in the cerebellum and the mesencephalic tegmentum. Mannitol 18 % 150 ml and 3 % hypertonic saline 250 ml were administered as emergency therapy, followed by the decision to insert an intraparenchymal transducer for continuous ICP monitoring (Codman, Pinewood Campus, UK) in the right frontal region, which recorded initial values around 30 mmHg. Thus, aggressive treatment of intracranial hypertension with continuous propofol infusion heavy sedation, hypertonic solutions, mild hyperventilation (PaCO2 between 30 and 32 mmHg), and use of vasopressors to maintain CPP levels ≥60 mmHg was started. With this treatment, a rapid and effective control of ICP was obtained (Fig. 2). However, after 36 h of relative stability, suddenly ICP rose again, rapidly approaching values above 35 mmHg despite conventional medical treatment. Brain magnetic resonance imaging showed a large extension of the edema (largely vasogenic), predominantly to the white matter of both cerebral hemispheres and ventricular effacement (Fig. 1g–l), associated with neuroradiological signs of tonsillar herniation (not shown). Thus, sodium thiopental bolus (375 mg) was immediately administered, followed by continuous infusion targeting burst suppression on continuous electroencephalogram. Cyclosporine and azathioprine were both discontinued and replaced with anti-thymocyte globulin. These treatments succeeded in achieving stable ICP reduction below 20 mmHg within 10 h (Fig. 2). During the following two days, barbiturate was rapidly tapered because of the emergence of urosepsis due to multidrug-resistant Acinetobacter baumannii. A total of 8.5 g of sodium thiopental was administered during the two-day schedule and then the patient was allowed to awake. The ICP catheter was removed after six days of monitoring, with no complications. Mycophenolate mofetil was started and anti-thymocyte globulin was withheld as scheduled after 5 days. On day 26 the patient regained consciousness and on day 50 she was discharged from the intensive care unit complaining of moderate headache and left lower limb hyposthenia, that resolved entirely over time. The magnetic resonance imaging abnormalities were almost resolved at 3 months, except for small alterations compatible with gliosis (Fig. 1m–r). The patient returned home after 3 months and scored Upper Moderate Disability in the Glasgow Outcome Scale Extended at 6 months, her major complaint being short-term amnesia.
Brain MRI of the patient. Initial MRI. Fluid-attenuated inversion recovery (FLAIR) MRI (a, b) showing bilateral hyperintense subcortical lesions in the temporo-parieto-occipital lobes and in both thalami; diffusion-weighted imaging (DWI) (c, d) and apparent diffusion coefficient (ADC) (e, f) maps of the lesions showing vasogenic edema in the subcortical white matter. Acute MRI. FLAIR (g, h), DWI (i, j), and ADC sequences (k, l) showing diffuse holohemispheric edema, mainly vasogenic. Follow-up MRI. FLAIR sequences (m, n) showing almost complete resolution of the brain lesions, with the exception of small FLAIR residual hyperintensities in the frontoparietal lobes and in left periventricular white matter, and corresponding DWI sequences and ADC maps (o–r)
Course of intracranial pressure (ICP), mean arterial pressure (MAP), and cerebral perfusion pressure (CPP). ICP, MAP, and CPP were continuously recorded and transmitted to a computer through an analog–digital converter (Mac Lab; ADInstruments Pty Ltd., Castle Hill, Australia) for storage and analysis. CPP was calculated on-line as the difference between MAP and ICP. Symbols represent off-line calculated mean hourly values of ICP, MAP, and CPP. The dashed line indicates the threshold for intracranial hypertension (20 mmHg). The shaded region corresponds to barbiturate infusion
Discussion
Recent studies on large series of patients with PRES confirm that it is reversible and its long-term outcome is favorable in up to 70–80 % of patients, provided that prompt discontinuation of potentially causative drugs and appropriate supportive therapy are undertaken [1]. However, it is also clear that a minority of subjects may die acutely or develop permanent lesions [6]. Death has been related to the extent of injuries, suggesting worse prognosis in patients with more diffuse lesions and with diffusion-weighted imaging and apparent diffusion coefficient patterns suggestive of cytotoxic edema [5].
In this case PRES was promptly recognized, tacrolimus was identified as the most likely drug that could have triggered it, and the patient was initially switched to cyclosporine. This strategy has been advocated by some authors in case of severe neurotoxicity following FK506, despite the neurotoxic profile of cyclosporine itself [7–12]. Moreover, sirolimus (a newer calcineurin inhibitor) was contraindicated in this patient, because it impairs wound healing, may cause bronchial airway dehiscence [13], and has itself recently been implicated in a case of PRES [14]. Nonetheless, the patient’s symptoms relapsed and progressed to a severe, life-threatening condition, which compelled us to discontinue all previously employed immunosuppressive drugs, and switch to a non-standard regimen with no reported neurotoxicity. This eventually turned into a clear clinical neurologic improvement, with no signs of graft rejection. Steroids were not considered as a likely precipitating drug, and they were, therefore, not discontinued, despite some evidence that they may cause PRES too [15]. The efficacy of steroids in reducing the cerebral edema associated with brain tumors is well established, but there is no clear demonstration of their usefulness as therapeutic agents in PRES. A high level of suspicion, careful monitoring for relapses, and thorough search for the precipitating drug(s) were likely more crucial to patient outcome than establishing the exact disease etiology.
However, the decision to institute ICP monitoring and aggressively treat refractory intracranial hypertension according to an ICP- and CPP-driven approach was probably equally life saving. Raised ICP may rapidly be fatal through brainstem compression and cerebral herniation, leading to irreversible brain damage and death if rapid measures to treat intracranial hypertension are not warranted. Although clear evidence is limited, the rationale for ICP monitoring and aggressive treatment in this particular case is that vasogenic edema may be completely reversible and, therefore, intracranial hypertension may respond to treatment. This is why we decided to undertake ICP monitoring after the second radiologic workup showed that most of the edema was still vasogenic and there was no evidence of major ischemic changes.
Much of the current practice of ICP monitoring has been derived from clinical experience with patients suffering from traumatic brain injury, which is one of the most studied indications for ICP monitoring [16]. On the contrary, ICP monitoring is not conventionally applied to treat non-neurosurgical conditions like PRES, and has only anecdotally been reported [17, 18]. Intracranial hypertension from diffuse brain edema in the context of PRES has been reported in an eclamptic young woman and in an otherwise healthy young woman, who were successfully and rapidly treated with osmotic therapy and sedation.
Treatment strategies of raised ICP in the context of fulminant hepatic failure and CNS infection have been applied in small series of patients but are still controversial [19–22]. The basic idea underlying such an approach is that raised ICP is deleterious and continuous monitoring is essential for appropriate treatment and for limiting side effects from undue “blinded” therapy for intracranial hypertension when ICP is not measured [23].
This is the first accurate description of a patient with PRES, in whom continuous ICP monitoring was instituted and aggressive second-tier treatment up to barbiturate coma was required to treat refractory intracranial hypertension. Notably, mild hyperventilation was achieved in this patient with no signs of ventilator-induced lung injury (VILI), and no other major systemic complications. At the present time, there are no indications on how to select patients for ICP monitoring outside traumatic brain injury. This case report suggests that patients with diffuse vasogenic edema and suspected intracranial hypertension may be considered for ICP monitoring, and that such an approach is feasible, safe, and possibly life saving in a potentially reversible disease.
Conclusions
This case shows that ICP monitoring may be indicated in PRES, when clinical and neuroimaging features show extensive vasogenic edema and suggest raised ICP, and that aggressive treatment of intracranial hypertension following an ICP- and CPP-driven approach can be life saving. High level of suspicion for this diagnosis, careful monitoring for relapses, and aggressive search for etiology seem equally crucial to patient outcome. At the present time, the incidence, extent, and therapeutic response of intracranial hypertension associated with PRES are still unclear, as well as the impact of raised ICP on long-term patient outcome.
References
Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol. 2012;259:155–64.
Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR. 2008;29:1043–9.
Prasad G, Gulati S, Gupta RK, Kumar R, Sharma K, Sharma RK. Is reversible posterior leukoencephalopathy with severe hypertension completely reversible in all patients? Pediatr Nephrol. 2003;18(11):1161–6.
Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR. 2008;29:1036–42.
Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR. 2002;23:1038–48.
Li Y, Gor D, Walicki D, et al. Spectrum and potential pathogenesis of reversible posterior leukoencephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21(8):873–82.
Filler G. Calcineurin inhibitors in pediatric renal transplant recipients. Paediatr Drugs. 2007;9:165–74.
Emre S, Genyk Y, Schluger LK, et al. Treatment of tacrolimus-related adverse effects by conversion to cyclosporine in liver transplant recipients. Transpl Int. 2000;13:73–8.
Jain A, Brody D, Hamad I, Rishi N, Kanal E, Fung J. Conversion to neoral for neurotoxicity after primary adult liver transplantation under tacrolimus. Transplantation. 2000;69(1):172–6.
Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13:313–26.
Wu Q, Marescaux C, Wolff V, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol. 2010;64:169–77.
Rosso L, Nosotti M, Mendogni P, et al. Lung transplantation and posterior reversible encephalopathy syndrome: a case series. Transplant Proc. 2012;44:2022–5.
Groetzner J, Kur F, Spelsberg F, et al. Airway anastomosis complications in de novo lung transplantation with sirolimus-based immunosuppression. J Heart Lung Transplant. 2004;23(5):632–8.
Moskowitz A, Nolan C, Lis E, Castro-Malaspina H, Perales MA. Posterior reversible encephalopathy syndrome due to sirolimus. Bone Marrow Transplant. 2007;39:653–4.
Pedraza R, Marik PE, Varon J. Posterior reversible encephalopathy syndrome: a review. Crit Care Shock. 2009;12:135–43.
Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37–44.
Fitzgerald-Hines J, King ML. Posterior reversible encephalopathy syndrome: a case study. J Neurosci Nurs. 2006;38:338–41.
Lee VH, Temes RE, John S, Conners JJ, Bleck T, Prabhakaran S. Posterior reversible leukoencephalopathy syndrome presenting with global cerebral edema and herniation. Neurocrit Care. 2013;18:81–3.
Vavilala MS. Intracranial pressure monitoring in meningitis: thinking beyond traumatic brain injury. Pediatr Crit Care Med. 2011;12(6):689–90.
Vavilala MS, Lam AM. Intraoperative intracranial pressure monitoring in pneumococcal meningitis. Anesth Analg. 2000;90:107–8.
Bacher A. Intracranial hypertension in fulminant hepatic failure. Transplant Proc. 2006;38:783–5.
Dunn LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002;73(Suppl 1):i23–7.
Stocchetti N. Evidence for intracranial pressure monitoring: a very demanding challenge. Neurosurgery. 2012;71(6):E1210–1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Facchini, A., Magnoni, S., Civelli, V. et al. Refractory Intracranial Hypertension in Posterior Reversible Encephalopathy Syndrome. Neurocrit Care 19, 376–380 (2013). https://doi.org/10.1007/s12028-013-9852-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-013-9852-z