Abstract
Background
Brain tissue oxygen monitoring (pBtO2) has been advocated in the treatment of patients with severe traumatic brain injuries (TBI); however, controversy exists regarding the improvements that pBtO2 monitoring provides. The objective of our study was to evaluate our experience and effect on mortality with goal directed pBtO2 monitoring for severe TBI compared to traditional ICP/CPP monitoring.
Methods
All patients admitted with severe TBI (GCS < 8) to our Level 1 trauma center from June 2007 through June 2009 were retrospectively analyzed. All patients had ICP monitoring and pBtO2 monitors were placed based on the current practices of the attending neurosurgeon producing two temporally matched cohorts of patients with and without pBtO2 monitors. Exclusion criteria were age <18 years and survival <24 h. Goal-directed therapy was utilized in all patients to maintain ICP <20 mmHg and CPP >60 mmHg. Patients with pBtO2 monitors were managed to maintain a level >20 mmHg.
Results
74 patients were treated for severe TBI over the 2-year study period with 37 patients in each group. Both groups were similar in age, sex, and admission Glascow Coma Score(GCS).The pBtO2-monitored group did, however, have significantly lower injury severity score [26 (25–30) vs. 30 (26–36), p = 0.03] and AIS Chest [0 (0–0) vs. 2 (0–3), p = 0.02]. There was no survival difference found (64.9 vs. 54.1 %, p = 0.34). No difference with respect to discharge GCS or discharge Functional Independence Measure score was identified.
Conclusions
Compared with ICP/CPP-directed therapy alone, the addition of pBtO2 monitoring did not provide a survival or functional status improvement at discharge. The true clinical benefit of pBtO2 monitoring will require further study.
Similar content being viewed by others
Introduction
Severe traumatic brain injury (TBI) accounts for significant morbidity and mortality each year in the United States. TBI is the cause of 40 % of trauma-related deaths, claiming approximately 52,000 lives each year. In addition to the safety efforts to avoid brain injury, much of the management of TBI has centered on the prevention of secondary insults related to edema, intra-cranial hypertension, cerebral hypoxia, and ischemia. Because mortality and poor functional outcome are closely linked to high intra-cranial pressure (ICP), the mainstay of treatment has been control of ICP and cerebral perfusion pressure (CPP) through the use of osmotic agents, vasopressors, ventilatory manipulation, drainage of cerebro spinal fluid (CSF), craniectomy, and barbiturate-induced coma. However, poor outcomes have been documented even in the setting of normal ICP and CPP values [1, 2]. This fact suggests that the ICP and CPP do not tell the whole story of brain tissue health.
Several other monitoring strategies have been developed in attempt to guide the management of patients with TBI and prevent secondary brain injury. One of these methods, which has received attention and support is brain tissue oxygen (pBtO2) monitoring. A number of observational studies have established a strong correlation between low pBtO2 values and poor patient outcomes [2–6]. More recently, several reports have attempted to prove the clinical benefit of pBtO2-directed therapy in TBI. Indeed, 2 studies comparing pBtO2-monitored patients to historic cohorts of ICP/CPP-monitored patients demonstrated significant improvements in the mortality rate and functional outcomes in the pBtO2-monitored patients [7, 8]. In contrast, two other studies using temporally matched cohorts failed to demonstrate statistically significant differences in mortality rate or functional outcomes between the two groups [9, 10].
In the present study, we attempted to determine the effect of pBtO2-directed therapy on mortality and functional outcome of patients with severe TBI at our Level 1 Trauma Center.
Methods
Monitoring and Patient Management
All the study patients were admitted to the surgical intensive care unit and co-managed by the Neurosurgical, Trauma, and Surgical Critical Care services according to the Brain Trauma Foundation Guidelines for the Management of Severe Traumatic Brain Injury (2007). [11].
All patients underwent intracranial pressure (ICP) monitoring. pBtO2 monitors (Licox Brain Tissue Oxygenation Probe, Integra NeuroSciences) were placed in non-injured brain tissue at the discretion of the attending neurosurgeon on call. All pBtO2 monitors were placed at the same time as ICP monitors were placed upon admission. The variability in current practices among neurosurgeons at our institution allowed for two temporally matched cohorts with or without pBtO2 monitoring; however, medical management was standardized and directed by the SICU attending. Monitors were removed after ICP measurements were less than 20 mmHg and pBtO2 levels were greater than 20 mHg for more than 24 h without intervention.
According to the established guidelines [14], therapy was directed in attempt to keep ICP <20 mmHg and CPP >60 mmHg in all patients. Initial ICP-lowering therapy involved patient positioning, normothermia (35–37 °C), drainage of CSF, maintenance of PaCO2 35–40 mmHg, sedation (Propfol), analgesia (Fentanyl), and administration of Mannitol (0.25–1 g/kg) if serum osmolarity was <320 mOsm. To improve CPP, IV fluids were used to optimize EDVI (80–120 mmHg), CVP (4–8 mmHg), and PCWP (8–12 mmHg). If CPP remained low, despite IV fluid administration, vasopressors were instituted to raise CPP >60 mmHg. Persistent elevation of ICP despite the above efforts was managed with intermittent hyperventilation, burst suppression with pentobarbital, and craniectomy.
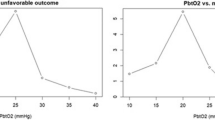
In addition to the above management of ICP, patients with pBtO2 monitors underwent therapies to maintain pBtO2 >20 mmHg. Initial management of low pBtO2 included optimization of ICP and CPP, a 100 % FiO2 challenge, breathing treatments, and adjustment of PaCO2 to 40–45 mmHg. Persistently depressed pBtO2 was managed by blood transfusion to obtain hemoglobin >10 g/dL, optimization of ventilator settings, and paralysis if needed to assist with hypoventilation.
The nursing protocol for monitoring TBI patients calls for hourly recording of ICP, pBtO2 values, and hemodynamic values. In addition, during acute changes in physiology nursing is encouraged to document additional time points in addition to hourly measurements.
Data Collection and Analysis
All patients with a diagnosis of severe traumatic brain injury (GCS ≤ 8) admitted to our Level 1 trauma center from June 2007 through June 2009 were identified through a prospectively collected observational database of trauma patients. This study was performed with the approval of the Institutional Review Board. Exclusion criteria for the study were age <18 years and survival <24 h. Electronic charts were reviewed to obtain data regarding patient demographics, severity of injury, hospital course, and condition at discharge. Serial clinical data, including hemodynamic measurements, respiratory monitoring, laboratory values, and measurements of intra-cranial pressure and brain tissue oxygenation were collected throughout the patients’ ICU courses. These were retrospectively analyzed and time-weighted averages were calculated assuming that data points were static between measurements. In order to time weight the ICP, CPP, MAP, heart rate, and pBtO2 values, a trailing time-biased approach was used to determine the area under the curve. The values were assumed to be static between time indexed points. Admission Glasgow Coma Score (GCS), Discharge GCS, Discharge Glasgow Outcome Score (GOS), Abbreviated Injury Severity Score (AIS), and Injury Severity Score (ISS) were recorded. In addition, Functional Independence Measure Score (FIMS) was recorded at discharge for survivors. This score evaluates the level of disability along three axes: feeding, expression, and locomotion. The score for each axis ranges from one, indicating full dependence on assistance, to four, indicating full independence [12, 13].
Normally distributed continuous variables are presented as mean ± SD. Non-normally distributed continuous variables are presented as median and 95 % confidence interval. Continuous variables were compared between groups by the 2-tailed T test. Categorical variables were compared between groups by the χ2-analysis. A p value <0.05 was used as a measure of statistical significance. SPSS version 13.0 (Chicago, IL, USA) was used for all statistical analyses.
Results
A total of 74 patients were admitted to Spectrum Health Butterworth Hospital with a diagnosis of severe traumatic brain injury during the study period. Thirty-seven patients had only an ICP monitor placed for monitoring; 37 had an ICP monitor and a pBtO2 monitor placed.
There was no statistically significant difference between the two groups with respect to age, gender, admission GCS, or AIS for head/neck, face, abdomen, pelvis, or extremities. In addition, there was no significant difference in type or severity of brain injury on initial head CT (Table 1). Patients in the pBtO2-monitored group had a lower admission ISS (26 vs. 30, p = 0.03) and lower AIS score for chest (0 vs. 2, p = 0.02) (Table 1).
Comparing time-weighted averages of clinical monitoring data revealed very little significant difference between the two groups. Patients managed according to brain tissue monitoring had significantly higher arterial oxygenation values throughout their ICU stays. Otherwise, all clinical data values were similar between the two groups (Table 2).
Primary endpoints of mortality and functional status at discharge did not differ significantly between the two study groups. Median length of hospitalization (19 days vs. 14 days, p = 0.02) and ICU stay (19 days vs. 10 days, p < 0.01) were significantly longer for the pBtO2-monitored group. In addition, pBtO2-monitored patients underwent craniectomy more frequently than ICP-only-monitored patients (18 vs. 9, p = 0.03 (Table 3).
Discussion
The prevention of secondary brain injury in TBI remains an elusive goal. In addition, pBtO2 values can be used as a predictor of mortality and functional outcome in TBI [2–6]. These facts have led some to adopt pBtO2-directed therapy as a means of secondary brain injury prevention.
In the present study, we did not observe a statistically significant benefit of pBtO2-directed therapy on the mortality for patients with severe TBI. These findings concur with several other recent observational studies. One study by McCarthy et al. [10] compared two temporally matched cohorts with and without pBtO2 monitors. Mortality rates were 31 % in the pBtO2-monitored group and 36 % in the ICP/CPP group (p = 0.52). Similarly, a study by Martini et al. [9] also compared two temporally matched cohorts with and without pBtO2 monitoring. Mortality rates were 29 % in the pBtO2-monitored group and 22 % in the ICP/CPP group (p = 0.12). In contrast, two reports of patients with pBtO2 monitors compared to historic controls by Narotam et al., and Spiotta et al. [7, 8] showed impressive mortality benefits of 25.9 vs. 41.5 % and 25.7 vs. 45.3 %, respectively. Comparisons between mortality in these studies is challenging, however, due to differences in patients and management protocols.
In the study by Spiotta et al., [8] patient management differed between the two groups. The authors indicate that when pBtO2 levels were adequate and CT scan did not reveal a mass lesion, they were more tolerant of mild elevations in ICP and depressions in CPP. This is supported by a trend toward higher average ICP and more episodes of CPP <60 mmHg in the pBtO2-monitored group. The authors go on to suggest that this tolerance may have resulted in less pulmonary complications, which are established side effects of aggressive CPP management with fluid boluses and administration of vasopressors [8]. Martini et al. and McCarthy et al. [9, 10] do not describe such a change in management in the pBtO2-monitored patients. There is evidence from their reported data that aggressive ICP and CPP management strategies were employed in pBtO2-monitored patients, just as in ICP/CPP-monitored patients [9, 10]. In the study by McCarthy et al. [10], there is a trend toward less time spent with an ICP >20 in the pBtO2-monitored group, and there is no significant difference between the two groups with respect to time spent with CPP <60. In the study by Martini et al. [9], there was a trend toward lower mean daily ICP in the pBtO2-monitored group. In addition, mannitol and hypertonic saline were used more frequently in the pBtO2-monitored group and there was a trend toward more frequent use of hyperventilation [9]. Although CPP values were not reported in this study, vasopressors were used more frequently in the pBtO2-monitored group [9]. These findings suggest aggressive management of ICP and CPP in the pBtO2-monitored group.
In the present study, all patients were managed aggressively to control ICP and CPP. The pBtO2 value was only used as an indication to add therapeutic measures over and above ICP/CPP management—not to de-escalate ICP/CPP therapies, as suggested by Spiotta et al. This change in management strategy may have an effect on overall mortality and functional outcomes in survivors. It has been shown that aggressive fluid resuscitation and administration of vasopressors can increase pulmonary complications in patients with TBI [14–16]. Avoidance of unnecessary hypertension and hypervolemia could be beneficial in patients with TBI.
In our cohort, surgical management also differed between the two groups. Patients with pBtO2 monitors were statistically more likely to undergo craniectomy. This group did have more epidural and subdural blood collections; however, this intervention may be associated with pBtO2 monitoring; however, the influence of surgeon bias cannot be excluded. Of the other four studies comparing pBtO2 monitoring, one showed an increased rate of craniectomy, one showed a decreased rate of craniectomy, one showed no difference in craniectomy, and one study did not comment on the craniectomy rate in each arm of the study [7–10].
Similar to mortality, results demonstrating improvement in functional recovery with pBtO2 monitoring have been mixed. We did not observe a statistically significant benefit of pBtO2-directed therapy functional outcome for patients with severe TBI based on both discharge GOS scores as well as FIM scores. Both the studies utilizing historic controls found an improvement in functional outcome for survivors [7, 8]. Similar to the findings in our cohort, McCarthy et al., [10] failed to demonstrate a significant functional outcome improvement for survivors in the concurrently managed pBtO2-monitored group. In addition, in Martini et al., [9], survivors in the pBtO2-monitored group had significantly less functional independence at discharge than the ICP/CPP group. The main weakness of this study was significant differences in patient age, ISS, and AIS Head between the two groups which may contribute to this lower functional independence [9]. Nanguroori et al. [17] performed a meta-analysis of these four studies and found improvement in mortality with pBtO2-based therapy. They called for a prospective study to confirm these findings.
Resource utilization has become an increasingly important issue in modern medicine. ICU LOS remains one of the highest costs for hospitalization for patients. We found patients treated with pBtO2 monitors had significantly more ICU and hospital days. This finding supports the findings of Spiotta et al., [8] who also demonstrated an increased ICU LOS. Two other reports, however, did not demonstrate this difference in length of stay in the ICU and hospital [9, 10]. Martini et al. [9] did, however, investigate hospital charges and did show a significant increase in charges for patients who were monitored with pBtO2 devices. The causes for increased charges and length of stay is often multifactorial, but must be considered when introducing new therapeutic agents and devises.
Our study has several potential limitations. This study carries with it all the limitations of a retrospectively generated dataset; however, the concurrent management of patients with and without pBtO2 monitors provides some advantages over utilizing historic controls. In addition, the number of subjects is limited and the results should be considered preliminary and are the most appropriately applied to clinical medicine as a hypothesis generating report. Although patient presentation and call schedules are often random, surgical preference, and decision making introduces a bias which cannot be fully accounted for in this project. The effect of selection in the creation of the two cohorts must be considered when evaluating the generalizability of this report. In addition, great debate exists regarding the correct monitor placement location and treatment threshold must be better characterized before undertaking a multicenter trial [6, 18]. Patients with TBI are known to have progressive recoveries over time, the choice of looking at discharge as the time point of comparison may be too early to determine long-term improved functional recovery in patients’ with pBtO2 monitors.
The multi-factorial nature of the care of TBI patients leads to the difficulty in assessing the effects of any single therapy. pBtO2-monitoring provides valuable prognostic information as well as a unique clinical data point which may be successfully manipulated [8, 19]. However, based on the findings of the present study and other similar studies, there is no clear survival benefit or improved functional outcome associated with pBtO2-monitoring using the current standard for pBtO2-directed therapy. The stark differences in mortality demonstrated by these five studies with two different methods of developing cohorts define a clear need for a large prospective multi-institutional study to determine the benefits and morbidity associated with pBtO2 monitoring.
References
van Santbrink H, vd Brink WA, Steyerberg EW, Carmona Suazo JA, Avezaat CJ, Maas AI. Brain tissue oxygen response in severe traumatic brain injury. Acta Neurochir (Wien). 2003;145:429–38. Discussion 38.
Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103:805–11.
Bardt TF, Unterberg AW, Hartl R, Kiening KL, Schneider GH, Lanksch WR. Monitoring of brain tissue PO2 in traumatic brain injury: effect of cerebral hypoxia on outcome. Acta Neurochir Suppl. 1998;71:153–6.
Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26:1576–81.
van den Brink WA, van Santbrink H, Steyerberg EW, et al. Brain oxygen tension in severe head injury. Neurosurgery. 2000;46:868–76. Discussion 76–8.
Eriksson EA, Barletta JF, Figueroa BE, et al. The first 72 hours of brain tissue oxygenation predicts patient survival with traumatic brain injury. J Trauma Acute Care Surg. 2012;72:1345–9.
Narotam PK, Morrison JF, Nathoo N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg. 2009;111:672–82.
Spiotta AM, Stiefel MF, Gracias VH, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg. 2010;113:571–80.
Martini RP, Deem S, Yanez ND, et al. Management guided by brain tissue oxygen monitoring and outcome following severe traumatic brain injury. J Neurosurg. 2009;111:644–9.
McCarthy MC, Moncrief H, Sands JM, et al. Neurologic outcomes with cerebral oxygen monitoring in traumatic brain injury. Surgery. 2009;146:585–90. Discussion 90–1.
Carney NA. Guidelines for the management of severe traumatic brain injury methods. J Neurotrauma. 2007;24(Suppl 1):S3–6.
Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996;77:1226–32.
Martin MJ, Mullenix PS, Steele SR, et al. Functional outcome after blunt and penetrating carotid artery injuries: analysis of the National Trauma Data Bank. J Trauma. 2005;59:860–4.
Coles JP, Minhas PS, Fryer TD, et al. Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates. Crit Care Med. 2002;30:1950–9.
Fletcher JJ, Bergman K, Blostein PA, Kramer AH. Fluid balance, complications, and brain tissue oxygen tension monitoring following severe traumatic brain injury. Neurocrit Care. 2010;13:47–56.
Robertson CS, Valadka AB, Hannay HJ, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27:2086–95.
Nangunoori R, Maloney-Wilensky E, Stiefel M, et al. Brain tissue oxygen-based therapy and outcome after severe traumatic brain injury: a systematic literature review. Neurocrit Care. 2012;17:131–8.
Rosenthal G, Hemphill JC III, Manley G. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2009;37:379–80.
Eriksson EA, Barletta JF, Figueroa BE, et al. Cerebral perfusion pressure and intracranial pressure are not surrogates for brain tissue oxygenation in traumatic brain injury. Clin Neurophysiol. 2011;123(6):1255–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, J.A., Pellegrini, D.C., Vanderkolk, W.E. et al. Goal Directed Brain Tissue Oxygen Monitoring Versus Conventional Management in Traumatic Brain Injury: An Analysis of In Hospital Recovery. Neurocrit Care 18, 20–25 (2013). https://doi.org/10.1007/s12028-012-9797-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-012-9797-7