Abstract
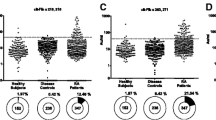
Although several infectious agents and particularly Epstein–Barr virus (EBV) have been suspected to be involved in aetiology of rheumatoid arthritis (RA), their role still remains elusive. Almost 80 % of RA sera contain antibodies to citrullinated proteins/peptides. Among them, the autoantibodies to citrullinated human fibrinogen (AhFibA) are composed of two non-cross-reactive subsets directed to immunodominant epitopes borne by the α36-50Cit and β60-74Cit fibrin peptides. RA sera also contain antibodies towards the citrullinated EBNA35-58Cit peptide derived from the EBNA-1 protein of EBV. Here, using a large cohort of RA patients and controls, we showed that for a diagnostic specificity of 98.5 %, 47 % of the AhFibA-positive patients were anti-EBNA35-58Cit-positive and that almost all (98.5 %) the anti-EBNA35-58Cit-positive were AhFibA-positive, whereas 86 % were anti-β60-74Cit-positive and only 43 % anti-α36-50Cit-positive. AhFibA, anti-EBNA35-58Cit- and anti-β60-74Cit-antibody titres were significantly correlated. Competition assays showed that anti-EBNA35-58Cit antibodies are highly cross-reactive with the β60-74Cit peptide. The demonstration that a citrullinated peptide derived from the EBNA-1 protein of EBV presents a molecular mimicry with human citrullinated fibrin constitutes an additional argument for a possible role of EBV in RA aetiopathogeny.




Similar content being viewed by others
References
Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36:182–8.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
van der Helm-van Mil AH, Wesoly JZ, Huizinga TW. Understanding the genetic contribution to rheumatoid arthritis. Curr Opin Rheumatol. 2005;17:299–304.
de Vries RR, Huizinga TW, Toes RE. Redefining the HLA and RA association: to be or not to be anti-CCP positive. J Autoimmun. 2005;25(Suppl):21–5.
Costenbader KH, Chang SC, De Vivo I, Plenge R, Karlson EW. Genetic polymorphisms in PTPN22, PADI-4, and CTLA-4 and risk for rheumatoid arthritis in two longitudinal cohort studies: evidence of gene-environment interactions with heavy cigarette smoking. Arthritis Res Ther. 2008;10:R52.
Pedersen M, Jacobsen S, Garred P, Madsen HO, Klarlund M, Svejgaard A, et al. Strong combined gene-environment effects in anti-cyclic citrullinated peptide-positive rheumatoid arthritis: a nationwide case-control study in Denmark. Arthritis Rheum. 2007;56:1446–53.
Becker HE, Vierbuchen C, Federlin K. Influence of Epstein–Barr virus infection on B lymphocyte responses in patients with rheumatoid arthritis. J Autoimmun. 1989;2:825–31.
Lotz M, Roudier J. Epstein–Barr virus and rheumatoid arthritis: cellular and molecular aspects. Rheumatol Int. 1989;9:147–52.
Toussirot E, Roudier J. Pathophysiological links between rheumatoid arthritis and the Epstein–Barr virus: an update. Joint Bone Spine. 2007;74:418–26.
McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution. Virus Res. 2006;117:90–104.
Kutok JL, Wang F. Spectrum of Epstein–Barr virus-associated diseases. Annu Rev Pathol. 2006;1:375–404.
Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404.
Thorley-Lawson DA. Epstein–Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82.
Klein G, Klein E, Kashuba E. Interaction of Epstein–Barr virus (EBV) with human B-lymphocytes. Biochem Biophys Res Commun. 2010;396:67–73.
Alspaugh MA, Jensen FC, Rabin H, Tan EM. Lymphocytes transformed by Epstein–Barr virus. Induction of nuclear antigen reactive with antibody in rheumatoid arthritis. J Exp Med. 1978;147:1018–27.
Billings PB, Hoch SO, White PJ, Carson DA, Vaughan JH. Antibodies to the Epstein–Barr virus nuclear antigen and to rheumatoid arthritis nuclear antigen identify the same polypeptide. Proc Natl Acad Sci USA. 1983;80:7104–8.
Baboonian C, Halliday D, Venables PJ, Pawlowski T, Millman G, Maini RN. Antibodies in rheumatoid arthritis react specifically with the glycine alanine repeat sequence of Epstein–Barr nuclear antigen-1. Rheumatol Int. 1989;9:161–6.
Catalano MA, Carson DA, Slovin SF, Richman DD, Vaughan JH. Antibodies to Epstein–Barr virus-determined antigens in normal subjects and in patients with seropositive rheumatoid arthritis. Proc Natl Acad Sci USA. 1979;76:5825–8.
Yokochi T, Yanagawa A, Kimura Y, Mizushima Y. High titer of antibody to the Epstein–Barr virus membrane antigen in sera from patients with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 1989;16:1029–32.
Balandraud N, Meynard JB, Auger I, Sovran H, Mugnier B, Reviron D, et al. Epstein–Barr virus load in the peripheral blood of patients with rheumatoid arthritis: accurate quantification using real-time polymerase chain reaction. Arthritis Rheum. 2003;48:1223–8.
Tosato G, Steinberg AD, Blaese RM. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981;305:1238–43.
Lunemann JD, Frey O, Eidner T, Baier M, Roberts S, Sashihara J, et al. Increased frequency of EBV-specific effector memory CD8+ T cells correlates with higher viral load in rheumatoid arthritis. J Immunol. 2008;181:991–1000.
Klatt T, Ouyang Q, Flad T, Koetter I, Buhring HJ, Kalbacher H, et al. Expansion of peripheral CD8+ CD28− T cells in response to Epstein–Barr virus in patients with rheumatoid arthritis. J Rheumatol. 2005;32:239–51.
Costenbader KH, Karlson EW. Epstein–Barr virus and rheumatoid arthritis: is there a link? Arthritis Res Ther. 2006;8:204.
Pratesi F, Tommasi C, Anzilotti C, Chimenti D, Migliorini P. Deiminated Epstein–Barr virus nuclear antigen 1 is a target of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:733–41.
Croia C, Serafini B, Bombardieri M, Kelly S, Humby F, Severa M, et al. Epstein–Barr virus persistence and infection of autoreactive plasma cells in synovial lymphoid structures in rheumatoid arthritis. Ann Rheum Dis. 2012;72:1559–68.
Sebbag M, Clavel C, Nogueira L, Arnaud J, Serre G. Autoimmune response to posttranslationally modified (citrullinated) proteins: prime suspect in the pathophysiology of rheumatoid arthritis. In: Zouali M, editor. The epigenetics of autoimmune diseases. Chichester:Wiley; 2009. p. 279–308. doi: 10.1002/9780470743553.
Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–6.
Nienhuis RL, Mandema E. A new serum factor in patients with rheumatoid arthritis; the antiperinuclear factor. Ann Rheum Dis. 1964;23:302–5.
Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–94.
Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81.
Masson-Bessière C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177–84.
Tilleman K, Van Steendam K, Cantaert T, De Keyser F, Elewaut D, Deforce D. Synovial detection and autoantibody reactivity of processed citrullinated isoforms of vimentin in inflammatory arthritides. Rheumatology (Oxford). 2008;47:597–604.
Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, et al. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1421–9.
Chapuy-Regaud S, Nogueira L, Clavel C, Sebbag M, Vincent C, Serre G. IgG subclass distribution of the rheumatoid arthritis-specific autoantibodies to citrullinated fibrin. Clin Exp Immunol. 2005;139:542–50.
Nielen MM, van der Horst AR, van Schaardenburg D, van der Horst-Bruinsma IE, van de Stadt RJ, Aarden L, et al. Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann Rheum Dis. 2005;64:1199–204.
Vander Cruyssen B, Cantaert T, Nogueira L, Clavel C, De Rycke L, Dendoven A, et al. Diagnostic value of anti-human citrullinated fibrinogen ELISA and comparison with four other anti-citrullinated protein assays. Arthritis Res Ther. 2006;8:R122.
Sebbag M, Moinard N, Auger I, Clavel C, Arnaud J, Nogueira L, et al. Epitopes of human fibrin recognized by the rheumatoid arthritis-specific autoantibodies to citrullinated proteins. Eur J Immunol. 2006;36:2250–63.
Cornillet M, Sebbag M, Verrouil E, Magyar A, Babos F, Ruyssen-Witrand A, et al. The fibrin-derived citrullinated peptide beta60-74Cit60,72,74 bears the major ACPA epitope recognised by the rheumatoid arthritis-specific anticitrullinated fibrinogen autoantibodies and anti-CCP2 antibodies. Ann Rheum Dis. 2014. doi:10.1136/annrheumdis-2012-202868.
Marchini B, Dolcher MP, Sabbatini A, Klein G, Migliorini P. Immune response to different sequences of the EBNA I molecule in Epstein–Barr virus-related disorders and in autoimmune diseases. J Autoimmun. 1994;7:179–91.
Merlini G, Anzilotti C, Chimenti D, Tommasi C, Bombardieri S, Migliorini P. A deiminated viral peptide to detect antibodies in rheumatoid arthritis. Ann N Y Acad Sci. 2005;1050:243–9.
Anzilotti C, Merlini G, Pratesi F, Tommasi C, Chimenti D, Migliorini P. Antibodies to viral citrullinated peptide in rheumatoid arthritis. J Rheumatol. 2006;33:647–51.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
Iobagiu C, Magyar A, Nogueira L, Cornillet M, Sebbag M, Arnaud J, et al. The antigen specificity of the rheumatoid arthritis-associated ACPA directed to citrullinated fibrin is very closely restricted. J Autoimmun. 2011;37:263–72.
McKenzie DP, Vida S, Mackinnon AJ, Onghena P, Clarke DM. Accurate confidence intervals for measures of test performance. Psychiatry Res. 1997;69:207–9.
Zhang J, Quan H, Ng J, Stepanavage ME. Some statistical methods for multiple endpoints in clinical trials. Control Clin Trials. 1997;18:204–21.
Pratesi F, Tommasi C, Anzilotti C, Puxeddu I, Sardano E, Di Colo G, et al. Antibodies to a new viral citrullinated peptide, VCP2: fine specificity and correlation with anti-cyclic citrullinated peptide (CCP) and anti-VCP1 antibodies. Clin Exp Immunol. 2011;164:337–45.
Ioan-Facsinay A, el-Bannoudi H, Scherer HU, van der Woude D, Menard HA, Lora M, et al. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann Rheum Dis. 2011;70:188–93.
Amara K, Steen J, Murray F, Morbach H, Fernandez-Rodriguez BM, Joshua V, et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med. 2013;210:445–55.
van de Stadt LA, van der Horst AR, de Koning MH, Bos WH, Wolbink GJ, van de Stadt RJ, et al. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann Rheum Dis. 2011;70:128–33.
Willemze A, Bohringer S, Knevel R, Levarht EW, Stoeken-Rijsbergen G, Houwing-Duistermaat JJ, et al. The ACPA recognition profile and subgrouping of ACPA-positive RA patients. Ann Rheum Dis. 2012;71:268–74.
Cai XM, Dass C. Conformational analysis of proteins and peptides. Curr Org Chem. 2003;7:1841–54.
Hermansson M, Artemenko K, Ossipova E, Eriksson H, Lengqvist J, Makrygiannakis D, et al. MS analysis of rheumatoid arthritic synovial tissue identifies specific citrullination sites on fibrinogen. Proteomics Clin Appl. 2010;4:511–8.
Tutturen AE, Fleckenstein B, de Souza GA. Assessing the citrullinome in rheumatoid arthritis synovial fluid with and without enrichment of citrullinated peptides. J Proteome Res. 2014;13:2867–73.
Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;. doi:10.1371/journal.pmed.0060001.
Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein–Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–11.
Uchida J, Yasui T, Takaoka-Shichijo Y, Muraoka M, Kulwichit W, Raab-Traub N, et al. Mimicry of CD40 signals by Epstein–Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–3.
Acknowledgments
The study was supported by research grants from the “Université of Toulouse”, the “Centre National de la Recherche Scientifique” (CNRS), the “Institut National de la Santé et de la Recherche Médicale” (INSERM) and the “Agence Nationale de la Recherche” (ANR : ANR-09-BLAN-0398). We thank Pr. B. Fournié (Centre de Rhumatologie, Hôpital Purpan, Toulouse), for providing patient sera. We also thank Marie-Françoise Isaïa, Axel Legué, Emilie Parra and Albano Lima Pérez for their excellent technical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cornillet, M., Verrouil, E., Cantagrel, A. et al. In ACPA-positive RA patients, antibodies to EBNA35-58Cit, a citrullinated peptide from the Epstein–Barr nuclear antigen-1, strongly cross-react with the peptide β60-74Cit which bears the immunodominant epitope of citrullinated fibrin. Immunol Res 61, 117–125 (2015). https://doi.org/10.1007/s12026-014-8584-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-014-8584-2