Abstract
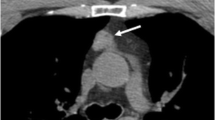
We describe a rare case of ectopic Cushing’s syndrome that recurred 6 years after resection of a thymic neuroendocrine carcinoma. We discuss reasons for the differing clinical presentations, management, hormone profiles, as well as immunopathology. A 41-year-old male developed acute-onset Cushing’s syndrome. Clinical presentation and laboratory results were compatible with ectopic adrenocorticotropin hormone (ACTH) production. Computerized tomography (CT) showed a 3.6 cm thymic tumor which was successfully resected. Plasma ACTH (P-ACTH) normalized the first postoperative day. Histopathology demonstrated a well-differentiated neuroendocrine carcinoma with diffuse positivity for ACTH and focal corticotropin-releasing hormone (CRH) reactivity in a few scattered cells. The patient was in remission for 6 years. He then again presented with acute-onset Cushing’s syndrome. Fluorine-labeled dihydroxyphenylalanine (18F-DOPA) PET/CT showed local uptake in the mediastinum and he underwent repeat resection. However, P-ACTH remained increased (613 ng/l) and 24-h urinary cortisol was 36,720 nmol, suggesting incomplete tumor removal or metastatic spread. Metyrapone treatment was initiated but then withdrawn because the patient spontaneously recovered and cortisol metabolism gradually normalized within 3 weeks. Histopathology demonstrated a recurrent neuroendocrine carcinoma with the same features as the previous lesion but this time CRH was strongly positive in more numerous cells. Normalization of P-ACTH after primary surgery was compatible with ectopic ACTH production. However, the delayed fall in P-ACTH and serum cortisol is compatible with ectopic CRH production and stimulation of pituitary ACTH secretion, which gradually resolved. Although ectopic CRH production is very rare, the unusual dynamics illustrated here should raise the possibility of CRH production by a neuroendocrine tumor.



Similar content being viewed by others
References
Alexandraki KI, Grossman AB. The ectopic ACTH syndrome. Rev Endocr Metab Disord 11:117–126, 2010.
Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing’s syndrome caused by ectopic ACTH secretion. Ann NY Acad Sci 970:134–144, 2007.
Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek H, Jenkins PJ, Monson JP, Grossman AB, Besser GM. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab 91:371–377, 2006.
Doi M, Sugiyama T, Izumiyama H, Yoshimoto T, Hirata Y. Clinical features and management of ectopic ACTH syndrome at a single institute in Japan. Endocr J 57:1061–1069; 2010.
Shahani S, Nudelman RJ, Nalini R, Kim H-S, Samson SL. Ectopic corticotropin-releasing hormone (CRH) syndrome from metastatic small cell carcinoma: a case report and review of the literature. Diagn Pathol 5:56, 2010.
Asa SL, Kovacs K, Vale W, Petrusz P, Vecsei P. Immunohistologic localization of corticotrophin-releasing hormone in human tumors. Am J Clin Pathol 87:327–333, 1987.
Schteingart DE, Lloyd RV, Akil H, Chandler WF, Ibarra-Perez G, Rosen SG, Ogletree R. Cushing’s syndrome secondary to ectopic corticotrophin-releasing hormone-adrenocorticotropin secretion. J Clin Endocrinol Metab 63:770–775, 1986.
Park SY, Rhee Y, Youn JC, Park YN, Lee S, Kim DM, Song SY, Lim S-K. Ectopic Cushing’s syndrome due to concurrent corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) secreted by malignant gastrinoma. Exp Clin Endocrinol Diabetes 144:444–447, 2007.
Markou A, Manning P, Kaya B, Datta SN, Bomanji JD, Conway GS. [18F]fluoro-2-deoxy-d-glucose ([18F]FDG) positron emission tomography imaging of thymic carcinoid tumor presenting with recurrent Cushing’s syndrome. Eur J Endocrinol 152:521–525, 2005.
Neary M, Lopez-Chavez A, Abel BS, Boyce AM, Schaub N, Kwong K, Stratakis CA, Moran CA, Giaccone G, Nieman LK (2012) Neuroendocrine ACTH-producing tumor of the thymus—experience with 12 patients over 25 years. J Clin Endocrinol Metab 97(7): 223–2230
Marx A, Shimosato Y, Kuo TT, Chan JKC, Travis WD, Wick MR. Thymic neuroendocrine tumours. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. World Health Organization Classification of tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press:188–195, 2004.
Kauhanen S, Seppänen M, Ovaska J, Minn H, Bergman J, Korsoff P, Salmela P, Saltevo J, Sane T, Välimäki M, Nuutila P. The clinical value of [18F]fluoro-dihydroxyphenylalanine positron emission tomography in primary diagnosis, staging, and restaging of neuroendocrine tumors. Endocr Relat Cancer 16:255–265, 2009.
Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. Am J Clin Pathol 114:100–110, 2000.
Eriksson B, Örlefors H, Öberg K, Sundin A, Bergström M, Långström B. Developments in PET for the detection of endocrine tumors. Best Pract Res Clin Endocrinol Metab 19:311–324, 2005.
Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastropancreatic neuroendocrine tumors: a meta-analysis. Endocrine 42:80–87, 2012.
Öberg K. Gallium-68 somatostatin receptor PET/CT: is it time to replace [111]Indium DTPA octreotide for patients with neuroendocrine tumors? Endocrine 42:3–4, 2012.
Schalin-Jäntti C, Ahonen A, Seppänen M. F-18-DOPA PET/CT but not Ga-68-DOTA-TOC PET/CT revealed the underlying cause of ectopic Cushing’s syndrome. Clin Nucl Med 37:904–905, 2012.
Sugiura H, Morikawa T, Itoh K, Ono K, Okushiba S, Kondo S. Katoh H. Thymic neuroendocrine carcinoma: a clinicopathologic study in four patients. Ann Thorac Cardiovasc Surg 6:304–308, 2000.
Acknowledgments
We thank Dr. Marko Seppänen at the Turku PET Centre, Turku University Hospital, Turku, Finland for providing the 18F-DOPA PET/CT images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schalin-Jäntti, C., Asa, S.L., Arola, J. et al. Recurrent Acute-Onset Cushing’s Syndrome 6 Years after Removal of a Thymic Neuroendocrine Carcinoma: From Ectopic ACTH to CRH. Endocr Pathol 24, 25–29 (2013). https://doi.org/10.1007/s12022-012-9228-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-012-9228-5