Abstract
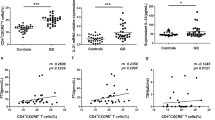
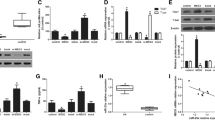
Follicular helper T (Tfh) cells are increasingly recognized as participants in various autoimmune diseases, including Graves’ disease. Although many transcription factors and cytokines are known to regulate Tfh cells, the role of noncoding RNA in Tfh cells development and function is poorly understood. Twenty-three patients with GD, eleven patients with remitting GD, and twenty-four healthy controls were enrolled in the current study. The interaction of miRNA and target gene was predicted through software analysis and then validated by luciferase assay and Western blot. The levels of miR-346 in circulating CD4+ T cells and plasma were measured by qRT-PCR. The correlation of miR-346 levels with the percentages of CD4+CXCR5+T cells and autoantibody levels were also analyzed. Up-regulation of Bcl-6 and down-regulation of miR-346 in GD patients were observed, and miR-346 could inhibit Bcl-6 at both transcriptional and translational levels. Overexpression of miR-346 led to attenuating CD4+CXCR5+ T cells. The abnormal expression of miR-346 restored in GD patients after treatment. A negative correlation between levels of miR-346 and percentages of CD4+CXCR5+ T cells was confirmed in GD patients. Additionally, negative correlations between the levels of miR-346 in circulating CD4+ T cells and serum concentrations of TR-Ab, TG-Ab, and TPO-Ab were also revealed in GD patients. MiR-346 regulates CD4+CXCR5+ T cells by targeting Bcl-6, a positive regulator of Tfh cells, and might play an important role in the pathogenesis of Graves’ disease.





Similar content being viewed by others
References
S. Crotty, Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011)
J. Rolf, S.E. Bell, D. Kovesdi, M.L. Janas, D.R. Soond, L.M. Webb, S. Santinelli, T. Saunders, B. Hebeis, N. Killeen, K. Okkenhaug, M. Turner, Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J. Immunol. 185(7), 4042–4052 (2010)
J. Ma, C. Zhu, B. Ma, J. Tian, S.E. Baidoo, C. Mao, W. Wu, J. Chen, J. Tong, M. Yang, Z. Jiao, H. Xu, L. Lu, S. Wang, Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2012, 827480 (2012)
S. Crotty, R.J. Johnston, S.P. Schoenberger, Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 11(2), 114–120 (2010)
R.J. Johnston, A.C. Poholek, D. DiToro, I. Yusuf, D. Eto, B. Barnett, A.L. Dent, J. Craft, S. Crotty, Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325(5943), 1006–1010 (2009)
R.I. Nurieva, Y. Chung, G.J. Martinez, X.O. Yang, S. Tanaka, T.D. Matskevitch, Y.H. Wang, C. Dong, Bcl6 mediates the development of T follicular helper cells. Science 325(5943), 1001–1005 (2009)
D. Yu, S. Rao, L.M. Tsai, S.K. Lee, Y. He, E.L. Sutcliffe, M. Srivastava, M. Linterman, L. Zheng, N. Simpson, J.I. Ellyard, I.A. Parish, C.S. Ma, Q.J. Li, C.R. Parish, C.R. Mackay, C.G. Vinuesa, The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31(3), 457–468 (2009)
M. Rotondi, L. Chiovato, Vitamin D deficiency in patients with Graves’ disease: probably something more than a casual association. Endocrine 43(1), 3–5 (2013)
A.P. Weetman, Autoimmune thyroid disease. Autoimmunity 37(4), 337–340 (2004)
E. Szczepanek-Parulska, M. Ruchala, A. Hernik, Unexpected conversion from hypothyroidism to an euthyroid state due to Graves’ disease in a patient with an ectopic thyroid. Endocrine 46(3), 684–685 (2014)
J. Zhang, H. Zeng, M. Ren, H. Yan, M. Xu, Z. Feng, W. Liang, C. Yang, H. Cheng, H. Ding, L. Yan, Interleukin-21 is associated with disease activity in patients with Graves’ disease. Endocrine 46(3), 539–548 (2014)
T. Kocjan, B. Wraber, U. Repnik, S. Hojker, Changes in Th1/Th2 cytokine balance in Graves’ disease. Pflugers Arch. 440(5 Suppl), R94–95 (2000)
Y. Nagayama, K. Watanabe, M. Niwa, S.M. McLachlan, B. Rapoport, Schistosoma mansoni and alpha-galactosylceramide: prophylactic effect of Th1 Immune suppression in a mouse model of Graves’ hyperthyroidism. J Immunol 173(3), 2167–2173 (2004)
T. Nanba, M. Watanabe, N. Inoue, Y. Iwatani, Increases of the Th1/Th2 cell ratio in severe Hashimoto’s disease and in the proportion of Th17 cells in intractable Graves’ disease. Thyroid 19(5), 495–501 (2009)
S. Wang, S.E. Baidoo, Y. Liu, C. Zhu, J. Tian, J. Ma, J. Tong, J. Chen, X. Tang, H. Xu, L. Lu, T cell-derived leptin contributes to increased frequency of T helper type 17 cells in female patients with Hashimoto’s thyroiditis. Clin. Exp. Immunol. 171(1), 63–68 (2013)
C. Zhu, J. Ma, Y. Liu, J. Tong, J. Tian, J. Chen, X. Tang, H. Xu, L. Lu, S. Wang, Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 97(3), 943–950 (2012)
D. Breitfeld, L. Ohl, E. Kremmer, J. Ellwart, F. Sallusto, M. Lipp, R. Forster, Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192(11), 1545–1552 (2000)
C.H. Kim, L.S. Rott, I. Clark-Lewis, D.J. Campbell, L. Wu, E.C. Butcher, Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 193(12), 1373–1381 (2001)
P. Schaerli, K. Willimann, A.B. Lang, M. Lipp, P. Loetscher, B. Moser, CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192(11), 1553–1562 (2000)
N. Fazilleau, L. Mark, L.J. McHeyzer-Williams, M.G. McHeyzer-Williams, Follicular helper T cells: lineage and location. Immunity 30(3), 324–335 (2009)
D.P. Bartel, MicroRNAs: target recognition and regulatory functions. Cell 136(2), 215–233 (2009)
J. Tian, K. Rui, S. Wang, Roles of miRNAs in regulating the differentiation and maturation of myeloid-derived suppressor cells. Med. Hypotheses 83(2), 151–153 (2014)
J. Lu, G. Getz, E.A. Miska, E. Alvarez-Saavedra, J. Lamb, D. Peck, A. Sweet-Cordero, B.L. Ebert, R.H. Mak, A.A. Ferrando, J.R. Downing, T. Jacks, H.R. Horvitz, T.R. Golub, MicroRNA expression profiles classify human cancers. Nature 435(7043), 834–838 (2005)
X. Tang, X. Tian, Y. Zhang, W. Wu, J. Tian, K. Rui, J. Tong, L. Lu, H. Xu, S. Wang, Correlation between the frequency of Th17 cell and the expression of MicroRNA-206 in patients with dermatomyositis. Clin. Dev. Immunol. 2013, 7 (2013)
T. Thum, C. Gross, J. Fiedler, T. Fischer, S. Kissler, M. Bussen, P. Galuppo, S. Just, W. Rottbauer, S. Frantz, M. Castoldi, J. Soutschek, V. Koteliansky, A. Rosenwald, M.A. Basson, J.D. Licht, J.T. Pena, S.H. Rouhanifard, M.U. Muckenthaler, T. Tuschl, G.R. Martin, J. Bauersachs, S. Engelhardt, MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456(7224), 980–984 (2008)
K.M. Pauley, M. Satoh, A.L. Chan, M.R. Bubb, W.H. Reeves, E.K. Chan, Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthr. Res. Ther. 10(4), R101 (2008)
E. Stagakis, G. Bertsias, P. Verginis, M. Nakou, M. Hatziapostolou, H. Kritikos, D. Iliopoulos, D.T. Boumpas, Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: miR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Ann. Rheum. Dis. 70(8), 1496–1506 (2011)
J. Tian, J. Ma, K. Ma, H. Guo, S.E. Baidoo, Y. Zhang, J. Yan, L. Lu, H. Xu, S. Wang, β-Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur. J. Immunol. 43(5), 1220–1230 (2013)
J.F. Chen, E.M. Mandel, J.M. Thomson, Q. Wu, T.E. Callis, S.M. Hammond, F.L. Conlon, D.Z. Wang, The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 38(2), 228–233 (2006)
A.P. Weetman, Graves’ disease. N. Engl. J. Med. 343(17), 1236–1248 (2000)
C. Du, C. Liu, J. Kang, G. Zhao, Z. Ye, S. Huang, Z. Li, Z. Wu, G. Pei, MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 10(12), 1252–1259 (2009)
K.P. Hoefig, V. Heissmeyer, MicroRNAs grow up in the immune system. Curr. Opin. Immunol. 20(3), 281–287 (2008)
C. Xiao, K. Rajewsky, MicroRNA control in the immune system: basic principles. Cell 136(1), 26–36 (2009)
E. Sonkoly, P. Janson, M.L. Majuri, T. Savinko, N. Fyhrquist, L. Eidsmo, N. Xu, F. Meisgen, T. Wei, M. Bradley, J. Stenvang, S. Kauppinen, H. Alenius, A. Lauerma, B. Homey, O. Winqvist, M. Stahle, A. Pivarcsi, MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J Allergy Clin Immunol. 126(3), 581–589 (2010). e581–520
Z. Feng, Y. Xia, M. Zhang, J. Zheng, MicroRNA-155 regulates T cell proliferation through targeting GSK3beta in cardiac allograft rejection in a murine transplantation model. Cell. Immunol. 281(2), 141–149 (2013)
X. Huang, X. Zhou, Z. Wang, F. Li, F. Liu, L. Zhong, X. Li, X. Han, Z. Wu, S. Chen, T. Zhao, CD99 triggers upregulation of miR-9-modulated PRDM1/BLIMP1 in Hodgkin/Reed-Sternberg cells and induces redifferentiation. Int. J. Cancer 131(4), E382–394 (2012)
J. Lin, T. Lwin, J.J. Zhao, W. Tam, Y.S. Choi, L.C. Moscinski, W.S. Dalton, E.M. Sotomayor, K.L. Wright, J. Tao, Follicular dendritic cell-induced microRNA-mediated upregulation of PRDM1 and downregulation of BCL-6 in non-Hodgkin’s B-cell lymphomas. Leukemia 25(1), 145–152 (2011)
Acknowledgments
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20133227110008), National Natural Science Foundation of China (Grant Nos. 31100648, 81072453), Specialized Project for Clinical Medicine of Jiangsu Province (Grant No. BL2014065), Health Department Foundation of Jiangsu Province (Grant No. Z201312), Science and technology support program (Social Development) of Zhenjiang (Grant Nos. SH2013040, SH2014039) Graduate Student Research and Innovation Program of Jiangsu Province (Grant Nos. CXZZ13_0700, KYLX_1074), Jiangsu Province “333” Project, and Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of interest
The authors have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Juan Chen, Jie Tian, and Xinyi Tang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, J., Tian, J., Tang, X. et al. MiR-346 regulates CD4+CXCR5+ T cells in the pathogenesis of Graves’ disease. Endocrine 49, 752–760 (2015). https://doi.org/10.1007/s12020-015-0546-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0546-5