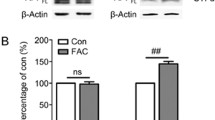
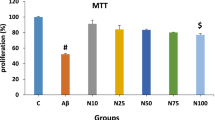
Abstract
The increased accumulation of iron in the brain in Alzheimer’s disease (AD) is well documented, and excess iron is strongly implicated in the pathogenesis of the disease. The adverse effects of accumulated iron in AD brain may include the oxidative stress, altered amyloid beta-metabolism and the augmented toxicity of metal-bound amyloid beta 42. In this study, we have shown that exogenously added iron in the form of ferric ammonium citrate (FAC) leads to considerable accumulation of amyloid precursor protein (APP) without a corresponding change in the concerned gene expression in cultured SHSY5Y cells during exposure up to 48 h. This phenomenon is also associated with increased β-secretase activity and augmented release of amyloid beta 42 in the medium. Further, the increase in β-secretase activity, in SHSY5Y cells, upon exposure to iron apparently involves reactive oxygen species (ROS) and NF-κB activation. The synthetic flavone negletein (5,6-dihydroxy-7-methoxyflavone), which is a known chelator for iron, can significantly prevent the effects of FAC on APP metabolism in SHSY5Y cells. Further, this compound inhibits the iron-dependent formation of ROS and also blocks the iron-induced oligomerization of amyloid beta 42 in vitro. In concentrations used in this study, negletein alone appears to have only marginal toxic effects on cell viability, but, on the other hand, the drug is capable of ameliorating the iron-induced loss of cell viability considerably. Our results provide the initial evidence of potential therapeutic effects of negletein, which should be explored in suitable animal models of AD.







Similar content being viewed by others
References
Aguirre, P., Mena, N., Tapia, V., Arredondo, M., & Nunez, M. T. (2005). Iron homeostasis in neuronal cells: A role for IREG1. BioMed Central Neuroscience. doi:10.1186/1471-2202-6-3.
Aracena, P., Aguirre, P., Munoz, P., & Nunez, M. T. (2009). Iron and glutathione at the crossroad of redox metabolism in neurons. Biological Research, 39, 157–165.
Bandyopadhyay, S., Cahill, C., Balleidier, A., Huang, C., Lahiri, D. K., Huang, X., et al. (2013). Novel 5′ untranslated region directed blockers of iron-regulatory protein-1 dependent amyloid precursor protein translation: Implications for down syndrome and Alzheimer’s disease. PLoS One, 8(7), e65978.
Baptista, F. I., Henriques, A. G., Silva, A. M., Wiltfang, J., da Cruz, E., & Silva, O. A. (2014). Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. ACS Chemical Neuroscience, 5(2), 83–92.
Barnham, K. J., Kenche, V. B., Ciccotosto, G. D., Smith, D. P., Tew, D. J., Liu, X., et al. (2008). Platinum-based inhibitors of amyloid-β as therapeutic agents for Alzheimer’s disease. Proceedings of the National Academy Sciences of the United States of America, 105(19), 6813–6818.
Beaudoin, M. E., Poirel, V.-J., & Krushel, L. A. (2008). Regulating amyloid precursor protein synthesis through an internal ribosomal entry site. Nucleic Acids Research, 36(21), 6835–6847.
Belyaev, N. D., Kellett, K. A., Beckett, C., Makova, N. Z., Revett, T. J., & Nalivaeva, N. N. (2010). The transcriptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a {beta}-secretase-dependent pathway. Journal of Biological Chemistry, 285(53), 41443–41454.
Bonda, D. J., Lee, H., Blair, J. A., Zhu, X., Perry, G., & Smith, M. A. (2011). Role of metal dyshomeostasis in Alzheimer’s disease. Metallomics, 3(3), 267–270.
Butterfield, D. A., Perluigi, M., Sultana, R., et al. (2006). Oxidative stress in Alzheimer’s disease brain: New insight from redox proteomics. European Journal of Pharmacology, 545(1), 39–50.
Chakrabarti, S., Sinha, M., Thakurta, I. G., Banerjee, P., & Chattopadhyay, M. (2013). Oxidative stress and amyloid beta toxicity in Alzheimer’s disease: Intervention in a complex relationship by antioxidants. Current Medicinal Chemistry, 20(37), 4648–4664.
Chami, L., & Checler, F. (2012). BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Molecular Neurodegeneration, 7, 52. doi:10.1186/1750-1326-7-52.
Chen, C. H., Zhou, W., Liu, S., Deng, Y., Cai, F., Tone, M., et al. (2012). Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. The International Journal of Neuropsychopharmacology, 15(1), 77–90.
Choi, D. Y., Lee, Y. J., Hong, J. T., & Lee, H. J. (2012). Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Research Bulletin, 87(2–3), 144–153.
Clark, J. B., Bates, T. E., Boakye, P., Kuimov, A., & Land, J. M. (1997). Investigation of mitochondrial defects in brain and skeletal muscle. In A. J. Turner & H. S. Bachelard (Eds.), Neurochemistry: A practical approach (pp. 151–174). New York: Oxford University Press Inc.
Commenges, D., Scotet, V., Renaud, S., Jacqmin-Gadda, H., Barberger-Gateau, P., & Dartigues, J. F. (2000). Intake of flavonoids and risk of dementia. European Journal of Epidemiology, 16(4), 357–363.
Dai, X., Sun, Y., Gao, Z., & Jiang, Z. (2010). Copper enhances amyloid-β peptide neuro-toxicity and non β-aggregation: A series of experiments conducted upon copper- bound and copper-free amyloid-β peptide. Journal of Molecular Neuroscience, 41(1), 66–73.
Dragicevic, N., Smith, A., Lin, X., Yuan, F., Copes, N., Delic, V., et al. (2011). Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. Journal of Alzheimer’s disease, 26(3), 507–521.
Duce, J. A., Bush, A. I., & Adlard, P. A. (2011). Role of amyloid-beta-metal interactions in Alzheimer’s disease. Future Neurology, 6(5), 641–659.
Guo, C., Wang, T., Zheng, W., Shan, Z. Y., Teng, W. P., & Wang, Z. Y. (2013a). Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiology of Aging, 34(2), 562–575.
Guo, C., Wang, P., Zhong, M. L., Wang, T., Huang, X. S., Li, J. Y., et al. (2013b). Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochemistry International, 62(2), 165–172.
Gutteridge, J. M. C. (1992). Iron and oxygen radicals in brain. Annals of Neurology, 32(S1), S16–S21.
Hallgren, B., & Sourander, P. (1958). The effect of age on the non-haemin iron in the human brain. Journal of Neurochemistry, 3(1), 41–51.
Halliwell, B., & Gutteridge, J. M. C. (1998). Free radicals in biology and medicine. Oxford: Oxford University Press.
Hayden, M. S., & Ghosh, S. (2004). Signaling to NF-kB. Genes and Development, 18(18), 2195–2224.
Hickok, J. R., Sahni, S., Mikhed, Y., Bonini, M. G., & Thomas, D. D. (2011). Nitric oxide suppresses tumor cell migration through N-Myc downstream-regulated gene-1 (NDRG1) expression role of chelatable iron. The Journal of Biological Chemistry, 286(48), 41413–41424.
Hoepken, H. H., Korten, T., Robinson, S. R., & Dringen, R. (2004). Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferring receptor in cultured astrocytes during incubation with ferric ammonium citrate. Journal of Neurochemistry, 88, 1194–1202.
Huang, X., Atwood, C. S., Moir, R. D., Hartshorn, M. A., Tanzi, R. E., & Bush, A. I. (2004). Trace metal contamination initiates the apparent auto-aggregation, amyloi- dosis, and oligomerization of Alzheimer’s Aβ peptides. Journal of Biological Inorganic Chemistry, 9(8), 954–960.
Hyman, B. T., Phelps, C. H., Beach, T. G., Bigio, E. H., Cairns, N. J., Carrillo, M. C., et al. (2012). National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers and Dementia, 8(1), 1–13.
Jana, S., Sinha, M., Chanda, D., Roy, T., Banerjee, K., Munshi, S., et al. (2011). Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: Implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochimica et Biophysica Acta, 1812(6), 663–673.
Jomova, K., Vondrakova, D., Lawson, M., & Valko, M. (2010). Metals, oxidative stress and neurodegenerative disorders. Molecular and Cellular Biochemistry, 345(1–2), 91–104.
Kanazawa, K., Uehara, M., Yanagitani, H., & Hashimoto, T. (2006). Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Archives of Biochemistry and Biophysics, 455(2), 2197–2203.
Khemka, V. K., Bagchi, D., Bandyopadhyay, K., Bir, A., Chattopadhyay, M., Biswas, A., et al. (2014). Altered serum levels of adipokines and insulin in probable Alzheimer’s disease. Journal of Alzheimers Disease. doi:10.3233/JAD-140006.
Li, Y. P., Bushnell, A. F., Lee, C. M., Perlmutter, L. S., & Wong, S. K. (1996). Beta-amyloid induces apoptosis in human-derived neurotypic SH-SY5Y cells. Brain Research, 738(2), 196–204.
Li, G., Zou, L. Y., Cao, C. M., & Yang, E. S. (2005). Coenzyme Q10 protects SHSY5Y neuronal cells from beta amyloid toxicity and oxygen-glucose deprivation by inhibiting the opening of the mitochondrial permeability transition pore. Biofactors, 25(1–4), 97–107.
Lin, Y.-Z., Yao, S. Y., Veach, R. A., Torgerson, T. R., & Hawiger, J. (1995). Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. The Journal of Biological Chemistry, 270(24), 14255–14258.
Lombardo, E., Sabellico, C., Hájek, J., Staňková, V., Filipský, T., Balducci, V., et al. (2013). Protection of cells against oxidative stress by nanomolar levels of hydroxyflavones indicates a new type of intracellular antioxidant mechanism. PLoS One, 8(4), e60796.
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L., & Markesbery, W. R. (1998). Copper, iron and zinc in Alzheimer’s disease senile plaques. Journal of the Neurological Sciences, 158(1), 47–52.
Macáková, K., Mladěnka, P., Filipský, T., Říha, M., Jahodář, L., Trejtnar, F., et al. (2012). Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chemistry, 135(4), 2584–2592.
Middleton, E, Jr, Kandaswami, C., & Theoharides, T. C. (2000). The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacological Reviews, 52(4), 673–751.
Mills, E., Dong, X.-P., Wang, F., & Xu, H. (2010). Mechanisms of brain iron transport: Insight into neurodegeneration and CNS disorders. Future Medicinal Chemistry, 2(1), 51–64.
Mladěnka, P., Macáková, K., Filipský, T., Zatloukalová, L., Jahodář, L., Bovicelli, P., et al. (2011). In vitro analysis of iron chelating activity of flavonoids. Journal of Inorganic Biochemistry, 105(5), 693–701.
Morel, Y., & Barouki, R. (1999). Repression of gene expression by oxidative stress. The Biochemical Journal, 342(3), 481–496.
Morgan, M. J., & Liu, Z-g. (2011). Crosstalk of reactive oxygen species and NF-κB signaling. Cell Research, 21(1), 103–115.
Mura, C. V., Delgado, R., Aguirre, P., Bacigalupo, J., & Núñez, M. T. (2006). Quiescence induced by iron challenge protects neuroblastoma cells from oxidative stress. Journal of Neurochemistry, 98(1), 11–19.
Nakamura, M., Shishido, N., Nunomura, A., Smith, M. A., Perry, G., Hayashi, Y., et al. (2007). Three histidine residues of amyloid-beta peptide control the redox activity of copper and iron. Biochemistry, 46(44), 12737–12743.
Olivieri, G., Baysang, G., Meier, F., Müller-Spahn, F., Stähelin, H. B., Brockhaus, M., et al. (2001a). N-acetyl-l-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: Effects on beta-amyloid secretion and tau phosphorylation. Journal of Neurochemistry, 76(1), 224–233.
Olivieri, G., Hess, C., Savaskan, E., Ly, C., Meier, F., Baysang, G., et al. (2001b). Melatonin protects SHSY5Y neuroblastoma cells from cobalt-induced oxidative stress, neurotoxicity and increased beta-amyloid secretion. Journal of Pineal Research, 31(4), 320–325.
Olivieri, G., Otten, U., Meier, F., Baysang, G., Dimitriades-Schmutz, B., Müller-Spahn, F., et al. (2003). Beta-amyloid modulates tyrosine kinase B receptor expression in SHSY5Y neuroblastoma cells: Influence of the antioxidant melatonin. Neuroscience, 120(3), 659–665.
Page, M., & Thorpe, R. (2002). Protein blotting by electroblotting. In J. M. Walker (Ed.), The protein protocols handbook (pp. 317–319). New Jersey: Humana Press.
Pfaffl, M. W. (2001). A new mathematical model for relative quantitative real-time RT-PCR. Nucleic Acids Research, 29(9), 2002–2007.
Prasanthi, J. R., Huls, A., Thomasson, S., Thompson, A., Schommer, E., & Ghribi, O. (2009). Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on β-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Molecular Neurodegeneration, 4, 1. doi:10.1186/1750-1326-4-1.
Prasanthi, J. R., Schrag, M., Dasari, B., Marwarha, G., Dickson, A., Kirsch, W. M., et al. (2012). Deferiprone reduces amyloid-β and tau phosphorylation levels but not reactive oxygen species generation in hippocampus of rabbits fed a cholesterol-enriched diet. Journal of Alzheimer’s Disease, 30(1), 167–182.
Procházková, D., Boušová, I., Wilhelmová, N., et al. (2011). Antioxidant and prooxidant properties of flavonoids. Fitoterapia, 82(4), 513–523.
Randall, C. N., Strasburger, D., Prozonic, J., Morris, S. N., Winkie, A. D., Parker, G. R., et al. (2009). Cluster analysis of risk factor genetic polymorphisms in Alzheimer’s disease. Neurochemical Research, 34(1), 23–28.
Reddy, P. H., & Beal, M. F. (2008). Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends in Molecular Medicine, 14(2), 45–53.
Riemer, J., Hoepken, H. H., Czerwinska, H., Robinson, S. R., & Dringen, R. (2004). Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Analytical Biochemistry, 331(2), 370–375.
Righi, G., Antonioletti, R., Silvestri, I. P., D’Antona, N., Lambusta, D., & Bovicelli, P. (2010). Convergent synthesis of mosloflavone, negletein and baicalein from crysin. Tetrahedron, 66(2010), 1294–1298.
Rogers, J. T., Randall, J. D., Cahill, C. M., Eder, P. S., Huang, X., Gunshin, H., et al. (2002). An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. The Journal of biological Chemistry, 277(47), 45518–45528.
Sambamurti, K., Kinsey, R., Maloney, B., Ge, Y. W., & Lahiri, D. K. (2004). Gene structure and organization of the human beta-secretase (BACE) promoter. Federation of American Societies for Experimental Biology Journal, 18, 1034–1036.
Sato, N., & Morishita, R. (2013). Roles of vascular and metabolic components in cognitive dysfunction of Alzheimer disease: Short- and long-term modification by non-genetic risk factors. Frontiers in Aging Neuroscience, 5(1), 64.
Sinha, M., Behera, P., Bhowmick, P., Banerjee, K., Basu, S., & Chakrabarti, S. (2011). Aging promotes amyloid-β peptide induced mitochondrial dysfunctions in rat brain: A molecular link between aging and Alzheimer’s disease. Journal of Alzheimer’s Disease, 27(4), 753–765.
Sinha, M., Bhowmick, P., Banerjee, A., & Chakrabarti, S. (2013). Antioxidant role of amyloid β protein in cell-free and biological systems: Implication for the pathogenesis of Alzheimer disease. Free Radical Biology and Medicine, 56(1), 184–192.
Smith, D. G., Cappai, R., & Barnham, K. J. (2007a). The redox chemistry of the Alzheimer’s disease amyloid b peptide. Biochimica et Biophysica Acta, 1768(8), 1976–1990.
Smith, D. P., Ciccotosto, G. D., Tew, D. J., Fodero-Tavoletti, M. T., Johanssen, T., & Masters, C. L. (2007b). Concentration dependent Cu2þ induced aggregation and dityrosine formation of the Alzheimer’s disease amyloid-b peptide. Biochemistry, 46(10), 2881–2891.
Smith, M. A., Harris, P. L. R., Sayre, L. M., & Perry, G. (1997). Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proceedings of the National Academy of Sciences of the United States of America, 94(18), 9866–9868.
Solano, D. C., Sironi, M., Bonfini, C., Solerte, S. B., Govoni, S., & Racchi, M. (2000). Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. Federation of American Societies for Experimental Biology Journal, 14(7), 1015–1022.
Swerdlow, R. H. (2007). Pathogenesis of Alzheimer’s disease. Clinical Interventions in Aging, 2(3), 347–359.
Symonowicz, M., & Kolanek, M. (2012). Flavonoids and their properties to form chelate complexes. Biotechnology and Food Science, 76(1), 35–41.
Thakurta, I. G., Chattopadhyay, M., Ghosh, A., & Chakrabarti, S. (2012). Dietary supplementation with N-acetyl cysteine, α-tocopherol and α-lipoic acid reduces the extent of oxidative stress and proinflammatory state in aged rat brain. Biogerontology, 13(5), 479–488.
Vanhoutte, G., Dewachter, I., Borghgraef, P., & Van Leuven, A. (2005). Non invasive in vivo MRI detection of neuritic plaques associated with iron in APP[V7171] transgenic mice, a model for Alzheimer’s disease. Magnetic Resonance in Medicine, 53(3), 607–613.
Wan, L., Nie, G., Zhang, J., Luo, Y., Zhang, P., & Zhang, Z., et al. (2011). β-Amyloid peptide increaes levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radical Biology & Medicine, 50(1), 122–129.
Xiong, Z., Hongmei, Z., Lu, S., & Yu, L. (2011). Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s Disease. Pharmacological Reports, 63(5), 1101–1108.
Zheng, L., Calvo-Garrido, J., Hallbeck, M., Hultenby, K., Marcusson, J., & Cedazo-Minguez, A. (2013). Intracellular localization of amyloid-β peptide in SH-SY5Y neuroblastoma cells. Journal of Alzheimer’s Disease, 37(4), 713–733.
Acknowledgments
The work was supported by a Grant from Department of Biotechnology, Govt. of India, New Delhi. PB was supported by a Senior Research Fellowship from Department of Science and Technology, Govt. of India, New Delhi.
Conflict of interest
The authors have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, P., Sahoo, A., Anand, S. et al. Multiple Mechanisms of Iron-Induced Amyloid Beta-Peptide Accumulation in SHSY5Y Cells: Protective Action of Negletein. Neuromol Med 16, 787–798 (2014). https://doi.org/10.1007/s12017-014-8328-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-014-8328-4