Abstract
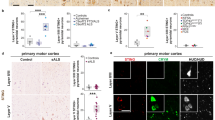
The NLRP3 inflammasome forms in response to a diverse range of stimuli and is responsible for the processing and release of interleukin-1β (IL-1β) from the immunocompetent cells of the brain. The pathological peptide of Alzheimer’s disease, amyloid beta (Aβ), induces formation of the NLRP3 inflammasome in a manner dependent on the family of proteases, cathepsins; however, the pathway by which cathepsins induce formation of the inflammasome has not yet been elucidated. In this study, we show that Aβ treatment of primary rat glial cultures increases cathepsin activation in the cytosol, formation of the NLRP3 inflammasome, caspase 1 activation and IL-1β release. We also show that a second NOD-like protein, NLRP10, is found bound to apoptosis-associated speck-like protein under resting conditions; however, with Aβ treatment, both in vitro and in vivo, NLRP10 is decreased. Further to these data, we show that cathepsins are capable of degrading NLRP10 and that treatment of glial cultures with recombinant NLRP10 reduces Aβ-induced caspase 1 activation and IL-1β release. We propose that Aβ-induced cathepsin released into the cytosol degrades NLRP10, thus allowing dissociation of NLRP3 and formation of the inflammasome.





Similar content being viewed by others
References
Bruchard, M., Mignot, G., Derangere, V., Chalmin, F., Chevriaux, A., Vegran, F., et al. (2012). Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nature Medicine. doi:10.1038/nm.2999.
Chu, J., Thomas, L. M., Watkins, S. C., Franchi, L., Nunez, G., & Salter, R. D. (2009). Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. The Journal of Immunology, 86(5), 1227–1238. doi:10.1189/jlb.0309164.
Chuang, S. Y., Yang, C. H., Chou, C. C., Chiang, Y. P., Chuang, T. H., & Hsu, L. C. (2013). TLR-induced PAI-2 expression suppresses IL-1β processing via increasing autophagy and NLRP3 degradation. Proceedings of the National Academy of Sciences of the United States of America, 110(40), 16079–16084.
Craft, J. M., Watterson, D. M., Hirsch, E., & Van Eldik, L. J. (2005). Interleukin 1 receptor antagonist knockout mice show enhanced microglial activation and neuronal damage induced by intracerebroventricular infusion of human beta-amyloid. Journal of Neuroinflammation, 2, 15. doi:10.1186/1742-2094-2-15.
Dostert, C., Petrilli, V., Van Bruggen, R., Steele, C., Mossman, B. T., & Tschopp, J. (2008). Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science, 320(5876), 674–677. doi:10.1126/science.1156995.
Duewell, P., Kono, H., Rayner, K. J., Sirois, C. M., Vladimer, G., Bauernfeind, F. G., et al. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature, 464(7293), 1357–1361.
Duncan, J. A., Gao, X., Huang, M. T., O’Connor, B. P., Thomas, C. E., Willingham, S. B., et al. (2009). Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. The Journal of Immunology, 182(10), 6460–6469. doi:10.4049/jimmunol.0802696.
Eisenbarth, S. C., Williams, A., Colegio, O. R., Meng, H., Strowig, T., Rongvaux, A., et al. (2012). NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature, 484(7395), 510–513. doi:10.1038/nature11012.
Frautschy, S. A., Hu, W., Kim, P., Miller, S. A., Chu, T., Harris-White, M. E., et al. (2001). Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiology of Aging, 22(6), 993–1005.
Halle, A., Hornung, V., Petzold, G. C., Stewart, C. R., Monks, B. G., Reinheckel, T., et al. (2008). The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunology, 9(8), 857–865. doi:10.1038/ni.1636.
Heneka, M. T., O’Banion, M. K., Terwel, D., & Kummer, M. P. (2010). Neuroinflammatory processes in Alzheimer’s disease. Journal of Neural Transmission, 117(8), 919–947. doi:10.1007/s00702-010-0438-z.
Hoegen, T., Tremel, N., Klein, M., Angele, B., Wagner, H., Kirschning, C., et al. (2011). The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. The Journal of Immunology, 187(10), 5440–5451. doi:10.4049/jimmunol.1100790.
Imamura, R., Wang, Y., Kinoshita, T., Suzuki, M., Noda, T., Sagara, J., et al. (2010). Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. The Journal of Immunology, 184(10), 5874–5884. doi:10.4049/jimmunol.0900779.
Lautz, K., Damm, A., Menning, M., Wenger, J., Adam, A. C., Zigrino, P., et al. (2012). NLRP10 enhances Shigella-induced pro-inflammatory responses. Cellular Microbiology, 14(10), 1568–1583. doi:10.1111/j.1462-5822.2012.01822.x.
Lyons, A., Downer, E. J., Crotty, S., Nolan, Y. M., Mills, K. H., & Lynch, M. A. (2007). CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: A role for IL-4. The Journal of Neuroscience, 27(31), 8309–8313. doi:10.1523/JNEUROSCI.1781-07.2007.
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A., & Tschopp, J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature, 440(7081), 237–241. doi:10.1038/nature04516.
Murphy, N., Cowley, T. R., Richardson, J. C., Virley, D., Upton, N., Walter, D., et al. (2011). The neuroprotective effect of a specific P2X(7) receptor antagonist derives from its ability to inhibit assembly of the NLRP3 inflammasome in glial cells. Brain Pathology. doi:10.1111/j.1750-3639.2011.00531.x.
Niemi, K., Teirila, L., Lappalainen, J., Rajamaki, K., Baumann, M. H., Oorni, K., et al. (2011). Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. The Journal of Immunology, 186(11), 6119–6128. doi:10.4049/jimmunol.1002843.
Nolan, Y., Martin, D., Campbell, V. A., & Lynch, M. A. (2004). Evidence of a protective effect of phosphatidylserine-containing liposomes on lipopolysaccharide-induced impairment of long-term potentiation in the rat hippocampus. Journal of Neuroimmunology, 151(1–2), 12–23. doi:10.1016/j.jneuroim.2004.02.001.
Ojala, J., Alafuzoff, I., Herukka, S. K., van Groen, T., Tanila, H., & Pirttila, T. (2009). Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiology of Aging, 30(2), 198–209. doi:10.1016/j.neurobiolaging.2007.06.006.
Rintahaka, J., Lietzen, N., Ohman, T., Nyman, T. A., & Matikainen, S. (2011). Recognition of cytoplasmic RNA results in cathepsin-dependent inflammasome activation and apoptosis in human macrophages. The Journal of Immunology, 186(5), 3085–3092. doi:10.4049/jimmunol.1002051.
Sardi, F., Fassina, L., Venturini, L., Inguscio, M., Guerriero, F., Rolfo, E., et al. (2011). Alzheimer’s disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmunity Reviews, 11(2), 149–153. doi:10.1016/j.autrev.2011.09.005.
Sastre, M., Walter, J., & Gentleman, S. M. (2008). Interactions between APP secretases and inflammatory mediators. Journal of Neuroinflammation, 5, 25. doi:10.1186/1742-2094-5-25.
Sheng, J. G., Mrak, R. E., & Griffin, W. S. (1997). Glial-neuronal interactions in Alzheimer disease: Progressive association of IL-1alpha+ microglia and S100beta+ astrocytes with neurofibrillary tangle stages. Journal of Neuropathology and Experimental Neurology, 56(3), 285–290.
Thomas, P. G., Dash, P., Aldridge, J. R, Jr., Ellebedy, A. H., Reynolds, C., Funk, A. J., et al. (2009). The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity, 30(4), 566–575. doi:10.1016/j.immuni.2009.02.006.
Wang, Y., Hasegawa, M., Imamura, R., Kinoshita, T., Kondo, C., Konaka, K., et al. (2004). PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1. International Immunology, 16(6), 777–786. doi:10.1093/intimm/dxh081.
Acknowledgments
The authors would like to thank the Irish Research Council and Science Foundation Ireland for funding this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murphy, N., Grehan, B. & Lynch, M.A. Glial Uptake of Amyloid Beta Induces NLRP3 Inflammasome Formation via Cathepsin-Dependent Degradation of NLRP10. Neuromol Med 16, 205–215 (2014). https://doi.org/10.1007/s12017-013-8274-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8274-6