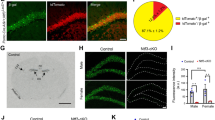
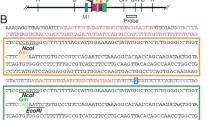
Abstract
Tomosyn, a syntaxin-binding protein, is known to inhibit vesicle priming and synaptic transmission via interference with the formation of SNARE complexes. Using a lentiviral vector, we specifically overexpressed tomosyn1 in hippocampal dentate gyrus neurons in adult mice. Mice were then subjected to spatial learning and memory tasks and electrophysiological measurements from hippocampal slices. Tomosyn1-overexpression significantly impaired hippocampus-dependent spatial memory while tested in the Morris water maze. Further, tomosyn1-overexpressing mice utilize swimming strategies of lesser cognitive ability in the Morris water maze compared with control mice. Electrophysiological measurements at mossy fiber-CA3 synapses revealed impaired paired-pulse facilitation in the mossy fiber of tomosyn1-overexpressing mice. This study provides evidence for novel roles for tomosyn1 in hippocampus-dependent spatial learning and memory, potentially via decreased synaptic transmission in mossy fiber-CA3 synapses. Moreover, it provides new insight regarding the role of the hippocampal dentate gyrus and mossy fiber-CA3 synapses in swimming strategy preference, and in learning and memory.





Similar content being viewed by others
References
Ashery, U., Bielopolski, N., et al. (2009). Friends and foes in synaptic transmission: The role of tomosyn in vesicle priming. Trends in Neuroscience, 32(5), 275–282.
Augustin, I., Korte, S., et al. (2001). The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice. The Journal of Neuroscience, 21(1), 10–17.
Baba, T., Sakisaka, T., et al. (2005). PKA-catalyzed phosphorylation of tomosyn and its implication in Ca2 + -dependent exocytosis of neurotransmitter. The Journal of cell biology, 170(7), 1113–1125.
Barak, B., Williams, A., et al. (2010). Tomosyn expression pattern in the mouse hippocampus suggests both presynaptic and postsynaptic functions. Frontiers in Neuroanatomy, 4, 149.
Bevins, R. A., & Besheer, J. (2006). Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nature Protocols, 1(3), 1306–1311.
Breustedt, J., Gundlfinger, A., et al. (2010). Munc13-2 differentially affects hippocampal synaptic transmission and plasticity. Cerebral Cortex, 20(5), 1109–1120.
Castillo, P. E., Janz, R., et al. (1997). Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature, 388(6642), 590–593.
Castillo, P. E., Schoch, S., et al. (2002). RIM1alpha is required for presynaptic long-term potentiation. Nature, 415(6869), 327–330.
Chen, K., Richlitzki, A., et al. (2011). Tomosyn-dependent regulation of synaptic transmission is required for a late phase of associative odor memory. Proceedings of the National Academy of Sciences of the United States of America, 108(45), 18482–18487.
D’Adamo, P., Wolfer, D. P., et al. (2004). Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. The European Journal of Neuroscience, 19(7), 1895–1905.
Day, L. B., Weisand, M., et al. (1999). The hippocampus is not necessary for a place response but may be necessary for pliancy. Behavioral Neuroscience, 113(5), 914–924.
de Almeida, L., Idiart, M., et al. (2010). The single place fields of CA3 cells: A two-stage transformation from grid cells. Hippocampus, 22(2), 200–208.
Fujita, Y., Shirataki, H., et al. (1998). Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron, 20(5), 905–915.
Gracheva, E. O., Burdina, A. O., et al. (2006). Tomosyn inhibits synaptic vesicle priming in caenorhabditis elegans. PLoS Biology, 4(8), e261.
Graziano, A., Petrosini, L., et al. (2003). Automatic recognition of explorative strategies in the Morris water maze. Journal of Neuroscience Methods, 130(1), 33–44.
Groffen, A. J., Jacobsen, L., et al. (2005). Two distinct genes drive expression of seven tomosyn isoforms in the mammalian brain, sharing a conserved structure with a unique variable domain. Journal of Neurochemistry, 92(3), 554–568.
Hagena, H., & Manahan-Vaughan, D. (2011). Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cerebral Cortex, 21(11), 2442–2449.
Hatsuzawa, K., Lang, T., et al. (2003). The R-SNARE motif of tomosyn forms SNARE core complexes with syntaxin 1 and SNAP-25 and down-regulates exocytosis. The Journal of Biological Chemistry, 278(33), 31159–31166.
Kjelstrup, K. B., Solstad, T., et al. (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143.
Kugler, S., Kilic, E., et al. (2003). Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Therapy, 10(4), 337–347.
Lee, I., & Kesner, R. P. (2002). Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nature Neuroscience, 5(2), 162–168.
Lee, I., & Kesner, R. P. (2003). Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behavioral Neuroscience, 117(5), 1044–1053.
Leutgeb, J. K., Leutgeb, S., et al. (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315(5814), 961–966.
Lonart, G., & Sudhof, T. C. (2000). Assembly of SNARE core complexes prior to neurotransmitter release sets the readily releasable pool of synaptic vesicles. The Journal of Biological Chemistry, 275(36), 27703–27707.
McHugh, T. J., & Tonegawa, S. (2009). CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus, 19(12), 1153–1158.
Migaud, M., Charlesworth, P., et al. (1998). Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature, 396(6710), 433–439.
Mohrmann, R., de Wit, H., et al. (2010). Fast vesicle fusion in living cells requires at least three SNARE complexes. Science, 330(6003), 502–505.
Morgan, A., Burgoyne, R. D., et al. (2005). Regulation of exocytosis by protein kinase C. Biochemical Society Transactions, 33(Pt 6), 1341–1344.
Morris, R. G., Garrud, P., et al. (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297(5868), 681–683.
Naldini, L., Blomer, U., et al. (1996). Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proceedings of the National Academy of Sciences of the United States of America, 93(21), 11382–11388.
Nofal, S., Becherer, U., et al. (2007). Primed vesicles can be distinguished from docked vesicles by analyzing their mobility. The Journal of Neuroscience, 27(6), 1386–1395.
Okun, E., Griffioen, K., et al. (2010). Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, 107(35), 15625–15630.
Powell, C. M. (2006). Gene targeting of presynaptic proteins in synaptic plasticity and memory: Across the great divide. Neurobiology of Learning and Memory, 85(1), 2–15.
Powell, C. M., Schoch, S., et al. (2004). The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron, 42(1), 143–153.
Rettig, J., & Neher, E. (2002). Emerging roles of presynaptic proteins in Ca ++-triggered exocytosis. Science, 298(5594), 781–785.
Richmond, J. E., & Broadie, K. S. (2002). The synaptic vesicle cycle: Exocytosis and endocytosis in Drosophila and C. elegans. Current Opinion in Neurobiology, 12(5), 499–507.
Rosenmund, C., Sigler, A., et al. (2002). Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron, 33(3), 411–424.
Saab, B. J., Saab, A. M., et al. (2011). Statistical and theoretical considerations for the platform re-location water maze. Journal of Neuroscience Methods, 198(1), 44–52.
Sakisaka, T., Yamamoto, Y., et al. (2008). Dual inhibition of SNARE complex formation by tomosyn ensures controlled neurotransmitter release. The Journal of Cell Biology, 183(2), 323–337.
Savelli, F., & Knierim, J. J. (2010). Hebbian analysis of the transformation of medial entorhinal grid-cell inputs to hippocampal place fields. Journal of Neurophysiology, 103(6), 3167–3183.
Si, B., & Treves, A. (2009). The role of competitive learning in the generation of DG fields from EC inputs. Cognitive Neurodynamics, 3(2), 177–187.
Silva, A. J., Rosahl, T. W., et al. (1996). Impaired learning in mice with abnormal short-lived plasticity. Current Biology, 6(11), 1509–1518.
Soderling, T. R., & Derkach, V. A. (2000). Postsynaptic protein phosphorylation and LTP. Trends in Neurosciences, 23(2), 75–80.
Sorensen, J. B., Matti, U., et al. (2002). The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proceedings of the National Academy of Sciences of the United States of America, 99(3), 1627–1632.
Sudhof, T. C. (2004). The synaptic vesicle cycle. Annual Review of Neuroscience, 27, 509–547.
Weimer, R. M., Richmond, J. E., et al. (2003). Defects in synaptic vesicle docking in unc-18 mutants. Nature Neuroscience, 6(10), 1023–1030.
Winters, B. D., Saksida, L. M., et al. (2010). Implications of animal object memory research for human amnesia. Neuropsychologia, 48(8), 2251–2261.
Wojcik, S. M., & Brose, N. (2007). Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron, 55(1), 11–24.
Wolfer, D. P., & Lipp, H. P. (2000). Dissecting the behaviour of transgenic mice: Is it the mutation, the genetic background, or the environment? Experimental Physiology, 85(6), 627–634.
Xu, W., Morishita, W., et al. (2012). Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron, 73(5), 990–1001.
Yamamoto, Y., Fujikura, K., et al. (2010). The tail domain of tomosyn controls membrane fusion through tomosyn displacement by VAMP2. Biochemical and Biophysical Research Communications 399(1): 24–30.
Yizhar, O., & Ashery, U. (2008). Modulating vesicle priming reveals that vesicle immobilization is necessary but not sufficient for fusion-competence. PLoS ONE, 3(7), e2694.
Yizhar, O., Lipstein, N., et al. (2007). Multiple functional domains are involved in tomosyn regulation of exocytosis. Journal of Neurochemistry, 103(2), 604–616.
Yizhar, O., Matti, U., et al. (2004). Tomosyn inhibits priming of large dense-core vesicles in a calcium-dependent manner. Proceedings of the National Academy of Sciences of the United States of America, 101(8), 2578–2583.
Yokoyama, S., Shirataki, H., et al. (1999). Three splicing variants of tomosyn and identification of their syntaxin-binding region. Biochemical and Biophysical Research Communications, 256(1), 218–222.
Acknowledgments
We would like to thank Prof. Pablo Castillo for comments on the manuscript, Dr. Peisu Zhang for technical help, and Dr. Sarah Rothman for editing the manuscript. This research was supported, in part, by the Intramural Research Program of the National Institute on Aging, NIH, the Israel Science Foundation (Grant no. 1211/07 and 730/11; U.A.) and the BSF (Grant no. 2009279; U.A.), the National Institutes of Health (RO1 NS053978; E.S. and U.A.) and by travel grant from Boehringer Ingelheim Fonds (awarded to Boaz Barak).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Boaz Barak and Eitan Okun: contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barak, B., Okun, E., Ben-Simon, Y. et al. Neuron-Specific Expression of Tomosyn1 in the Mouse Hippocampal Dentate Gyrus Impairs Spatial Learning and Memory. Neuromol Med 15, 351–363 (2013). https://doi.org/10.1007/s12017-013-8223-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8223-4