Abstract
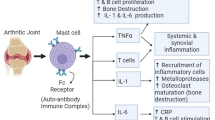
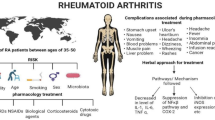
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune inflammatory disorder that causes permanent disability and mortality to approximately 1 to 100 people in the world. Patients with RA not only suffer from pain, stiffness, swelling, and loss of function in their joints, but also have a higher risk of cardiovascular disease and lymphoma. Typically prescribed medications, including pain-relieving drugs, nonsteroidal anti-inflammatory drugs (NSAID), and disease-modifying antirheumatic drugs, can help to relieve pain, reduce inflammation and slow the course of disease progression in RA patients. However, the general effectiveness of the drugs has been far from satisfactory. Other therapeutic modalities like TNF-alpha (TNF-α) inhibitors and interleukin-1 receptor antagonists targeting precise pathways within the immune system are expensive and may be associated with serious side effects. Recently, botanical medicines have become popular as alternative remedies as they are believed to be efficacious, safe and have over a thousand years experience in treating patients. In this review, we will summarize recent evidence for pharmacological effects of herbs including Black cohosh, Angelica sinensis, Licorice, Tripterygium wilfordii, Centella asiatica, and Urtica dioica. Scientific research has demonstrated that these herbs have strong anti-inflammatory and anti-arthritic effects. A wide range of phytochemicals including phenolic acids, phenylpropanoid ester, triterpene glycosides, phthalide, flavonoids, triterpenoid saponin, diterpene and triterpene have been isolated and demonstrated to be responsible for the biological effects of the herbs. Understanding the mechanisms of action of the herbs may provide new treatment opportunities for RA patients.



Similar content being viewed by others
References
Myasoedova E, Crowson CS, Turesson C, Gabriel SE, Matteson EL (2011) Incidence of extraarticular rheumatoid arthritis in olmsted county, minnesota, in 1995-2007 versus 1985-1994: a population-based study. J Rheumatol 38:983–989
Aletaha D, Neogi T, Silman AJ et al (2010) 2010 rheumatoid arthritis classification criteria an american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum-Us 62:2569–2581
Nordahl S, Alstergren P, Kopp S (2000) Tumor necrosis factor-alpha in synovial fluid and plasma from patients with chronic connective tissue disease and its relation to temporomandibular joint pain. J Oral Maxil Surg 58:525–530
Nawroth PP, Bank I, Handley D, Cassimeris J, Chess L, Stern D (1986) Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med 163:1363–1375
Suzuki M, Tetsuka T, Yoshida S et al (2000) The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-alpha- or IL-1beta-stimulated rheumatoid synovial fibroblasts. FEBS Lett 465:23–27
Haworth C, Brennan FM, Chantry D, Turner M, Maini RN, Feldmann M (1991) Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-alpha. Eur J Immunol 21:2575–2579
Chin JE, Winterrowd GE, Krzesicki RF, Sanders ME (1990) Role of cytokines in inflammatory synovitis. The coordinate regulation of intercellular adhesion molecule 1 and HLA class I and class II antigens in rheumatoid synovial fibroblasts. Arthritis Rheum 33:1776–1786
Cunnane G, Hummel KM, Muller-Ladner U, Gay RE, Gay S (1998) Mechanism of joint destruction in rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 46:1–7
Shingu M, Nagai Y, Isayama T, Naono T, Nobunaga M, Nagai Y (1993) The effects of cytokines on metalloproteinase inhibitors (timp) and collagenase production by human chondrocytes and timp production by synovial-cells and endothelial-cells. Clin Exp Immunol 94:145–149
Nagy G, Clark JM, Buzas E et al (2008) Nitric oxide production of T lymphocytes is increased in rheumatoid arthritis. Immunol Lett 118:55–58
Butler DM, Maini RN, Feldmann M, Brennan FM (1995) Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur. Cytokine Netw 6:225–230
Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW (1988) Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet 2:706–709
Amin AR, Dave M, Attur M, Abramson SB (2000) COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep 2:447–453
Chabaud M, Durand JM, Buchs N et al (1999) Human interleukin-17 - A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 42:963–970
Jovanovic DV, Di Battista JA, Martel-Pelletier J et al (1998) IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol 160:3513–3521
LeGrand A, Fermor B, Fink C et al (2001) Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E-2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum 44:2078–2083
Jovanovic DV, Martel-Pelletier J, Di Battista JA et al (2000) Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophages - a possible role in rheumatoid arthritis. Arthritis Rheum 43:1134–1144
Pickens SR, Volin MV, Mandelin AM, Kolls JK, Pope RM, Shahrara S (2010) IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol 184:3233–3241
Yoshihara Y, Nakamura H, Obata K et al (2000) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis 59:455–461
Burrage PS, Mix KS, Brinckerhoff CE (2006) Matrix metalloproteinases: role in arthritis. Front Biosci 11:529–543
Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G (1996) Biochemical characterization of human collagenase-3. J Biol Chem 271:1544–1550
Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ (1996) Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett 380:17–20
Okada Y, Nagase H, Harris ED Jr (1986) A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purif charact J Biol Chem 261:14245–14255
Flannery CR, Lark MW, Sandy JD (1992) Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem 267:1008–1014
Conway JG, Wakefield JA, Brown RH et al (1995) Inhibition of cartilage and bone destruction in adjuvant arthritis in the rat by a matrix metalloproteinase inhibitor. J Exp Med 182:449–457
Hishinuma T, Nakamura H, Sawai T, Uzuki M, Itabash Y, Mizugaki M (1999) Microdetermination of prostaglandin E2 in joint fluid in rheumatoid arthritis patients using gas chromatography/selected ion monitoring. Prostaglandins Lipid Mediat 58:179–186
Ferreira SH (1972) Prostaglandins, aspirin-like drugs and analgesia. Nature-New Biol 240: 200-&
Lemos HP, Grespan R, Vieira SM et al (2009) Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFN gamma production. P Natl Acad Sci USA 106:5954–5959
Akaogi J, Nozaki T, Satoh M, Yamada H (2006) Role of PGE2 and EP receptors in the pathogenesis of rheumatoid arthritis and as a novel therapeutic strategy. Endocr Metab Immune Disord Drug Targets 6:383–394
Campbell IK, Piccoli DS, Hamilton JA (1990) Stimulation of human chondrocyte prostaglandin E2 production by recombinant human interleukin-1 and tumour necrosis factor. Biochim Biophys Acta 1051:310–318
Sakurai H, Kohsaka H, Liu MF et al (1995) Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest 96:2357–2363
Ajuebor MN, Virag L, Flower RJ, Perretti M, Szabo C (1998) Role of inducible nitric oxide synthase in the regulation of neutrophil migration in zymosan-induced inflammation. Immunology 95:625–630
Lotz M (1999) The role of nitric oxide in articular cartilage damage. Rheum Dis Clin N Am 25:269–282
Ralston SH, Ho LP, Helfrich MH, Grabowski PS, Johnston PW, Benjamin N (1995) Nitric-oxide - a cytokine-induced regulator of bone-resorption. J Bone Miner Res 10:1040–1049
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423:356–361
Maruotti N, Cantatore P, Crivellato E, Vacca A, Ribatti D (2007) Macrophages in rheumatoid arthritis. Histol Histopathol 22:581–586
Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR (2000) Macrophages in rheumatoid arthritis. Arthritis Research 2:189–202
Goldring SR (2003) Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology 42:11–16
Silverman GJ, Carson DA (2003) Roles of B cells in rheumatoid arthritis. Arthritis Res Ther 5:S1–S6
Jones DH, Kong YY, Penninger JM (2002) Role of RANKL and RANK in bone loss and arthritis. Ann Rheum Dis 61:32–39
Dayer JM, Burger D (1999) Cytokines and direct cell contact in synovitis: relevance to therapeutic intervention. Arthritis Res 1:17–20
Burger D, Rezzonico R, Li JM et al (1998) Imbalance between interstitial collagenase and tissue inhibitor of metalloproteinases 1 in synoviocytes and fibroblasts upon direct contact with stimulated T lymphocytes - Involvement of membrane-associated cytokines. Arthritis Rheum 41:1748–1759
Schulzekoops H, Lipsky PE, Kavanaugh AF, Davis LS (1995) Elevated Th1- or Th0-like cytokine messenger-rna in peripheral-circulation of patients with rheumatoid-arthritis - modulation by treatment with anti-icam-1 correlates with clinical benefit. J Immunol 155:5029–5037
van Hamburg JP, Asmawidjaja PS, Davelaar N et al (2011) Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum 63:73–83
Schett G, Tohidast-Akrad M, Smolen JS et al (2000) Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum 43:2501–2512
Brauchle M, Gluck D, Di Padova F, Han J, Gram H (2000) Independent role of p38 and ERK1/2 mitogen-activated kinases in the upregulation of matrix metalloproteinase-1. Exp Cell Res 258:135–144
Westermarck J, Holmstrom T, Ahonen M, Eriksson JE, Kahari VM (1998) Enhancement of fibroblast collagenase-1 (MMP-1) gene expression by tumor promoter okadaic acid is mediated by stress-activated protein kinases Jun N-terminal kinase and p38. Matrix Biol 17:547–557
Han ZN, Boyle DL, Manning AM, Firestein GS (1998) AP-1 and NF-kappa B regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity 28:197–208
Yan C, Boyd DD (2007) Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211:19–26
Miyazawa K, Mori A, Yamamoto K, Okudaira H (1998) Transcriptional roles of CCAAT/enhancer binding protein-beta, nuclear factor-kappaB, and C-promoter binding factor 1 in interleukin (IL)-1beta-induced IL-6 synthesis by human rheumatoid fibroblast-like synoviocytes. J Biol Chem 273:7620–7627
Nishiya T, Uehara T, Kaneko M, Nomura Y (2000) Involvement of nuclear factor-kappaB (NF-kappaB) signaling in the expression of inducible nitric oxide synthase (iNOS) gene in rat C6 glioma cells. Biochem Biophys Res Commun 275:268–273
Kopp EB, Ghosh S (1995) NF-kappa B and rel proteins in innate immunity. Adv Immunol 58:1–27
Moreland LW, Baumgartner SW, Schiff MH et al (1997) Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. New Engl J Med 337:141–147
Brennan FM, Jackson A, Chantry D, Maini R, Feldmann M (1989) Inhibitory effect of tnf-alpha antibodies on synovial cell interleukin-1 production in rheumatoid-arthritis. Lancet 2:244–247
Smolen JS, Landewe R, Breedveld FC et al (2010) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 69:964–975
Smolen JS, Avila JC, Aletaha D (2011) Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: disassociation of the link between inflammation and destruction. Ann Rheum Dis. doi:10.1136/annrheumdis-2011-200395
De Vita S, Zaja F, Sacco S, De Candia A, Fanin R, Ferraccioli G (2002) Efficacy of selective B cell blockade in the treatment of rheumatoid arthritis: evidence for a pathogenetic role of B cells. Arthritis Rheum 46:2029–2033
Blumenthal MG, Brinkman J (eds) (2000) Black cohosh root. In herbal medicine: the expanded commission e monographs. American Botanical Council, Newton, pp 22–27
Schmid D, Gruber M, Woehs F et al (2009) Inhibition of inducible nitric oxide synthesis by Cimicifuga racemosa (Actaea racemosa, black cohosh) extracts in LPS-stimulated RAW 264.7 macrophages. J Pharm Pharmacol 61:1089–1096
Yang CLH, Chik SCC, Li JCB, Cheung BKW, Lau ASY (2009) Identification of the bioactive constituent and its mechanisms of action in mediating the anti-inflammatory effects of black cohosh and related cimicifuga species on human primary blood macrophages. J Med Chem 52:6707–6715
Schmid D, Woehs F, Svoboda M, Thalhammer T, Chiba P, Moeslinger T (2009) Aqueous extracts of Cimicifuga racemosa and phenolcarboxylic constituents inhibit production of proinflammatory cytokines in LPS-stimulated human whole blood. Can J Physiol Pharmacol 87:963–972
Sakai S, Ochiai H, Nakajima K, Terasawa K (1997) Inhibitory effect of ferulic acid on macrophage inflammatory protein-2 production in a murine macrophage cell line, RAW264.7. Cytokine 9:242–248
Hirabayashi T, Ochiai H, Sakai S, Nakajima K, Terasawa K (1995) Inhibitory effect of ferulic acid and isoferulic acid on murine interleukin-8 production in response to influenza-virus infections in-vitro and in-vivo. Planta Med 61:221–226
Hirata A, Murakami Y, Atsumi T et al (2005) Ferulic acid dimer inhibits lipopolysaccharide-stimulated cyclooxygenase-2 expression in macrophages. In Vivo 19:849–853
Qiu SX, Dan C, Ding LS et al (2007) A triterpene glycoside from black cohosh that inhibits osteoclastogenesis by modulating RANKL and TNFalpha signaling pathways. Chem Biol 14:860–869
Chawla AS, Singh M, Murthy MS, Gupta M, Singh H (1987) Anti-inflammatory action of ferulic acid and its esters in carrageenan induced rat paw oedema model. Indian J Exp Biol 25:187–189
Mills SY, Jacoby RK, Chacksfield M, Willoughby M (1996) Effect of a proprietary herbal medicine on the relief of chronic arthritic pain: a double-blind study. Brit J Rheumatol 35:874–878
Soeken KL, Miller SA, Ernst E (2003) Herbal medicines for the treatment of rheumatoid arthritis: a systematic review. Rheumatology 42:652–659
Hou TC (ed) (2004) Herbal extracts, Vols. 1, 1st edn. China Medical Scientific Technological Publishing Company, Beijing, pp 173–183
Chen MP, Yang SH, Chou CH et al (2010) The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm Res 59:587–595
Zhang RX, Fan AY, Zhou AN et al (2009) Extract of the Chinese herbal formula Huo Luo Xiao Ling Dan inhibited adjuvant arthritis in rats. J Ethnopharmacol 121:366–371
Chao WW, Kuo YH, Li WC, Lin BF (2009) The production of nitric oxide and prostaglandin E(2) in peritoneal macrophages is inhibited by Andrographis paniculata, Angelica sinensis and Morus alba ethyl acetate fractions. J Ethnopharmacol 122:68–75
Yang C, Niu S, Yu L, Zhu S, Zhu J, Zhu Q (2011) The aqueous extract of angelica sinensis, a popular chinese herb, inhibits wear debris-induced inflammatory osteolysis in mice. J Surg Res
Chao WW, Hong YH, Chen ML, Lin BF (2010) Inhibitory effects of Angelica sinensis ethyl acetate extract and major compounds on NF-kappa B trans-activation activity and LPS-induced inflammation. J Ethnopharmacol 129:244–249
Chu Q, Hashimoto K, Satoh K, Wang QT, Sakagami H (2009) Effect of three herbal extracts on NO and PGE(2) production by activated mouse macrophage-like cells. In Vivo 23:537–544
Fu RH, Hran HJ, Chu CL et al (2011) Lipopolysaccharide-stimulated activation of murine DC2.4 cells is attenuated by n-butylidenephthalide through suppression of the NF-kappaB pathway. Biotechnol Lett 33:903–910
Ozaki Y (1992) Antiinflammatory effect of tetramethylpyrazine and ferulic acid. Chem Pharm Bull 40:954–956
Ju XD, Deng M, Ao YF et al (2010) The protective effect of tetramethylpyrazine on cartilage explants and chondrocytes. J Ethnopharmacol 132:414–420
Yeh JC, Cindrova-Davies T, Belleri M et al (2011) The natural compound n-butylidenephthalide derived from the volatile oil of Radix Angelica sinensis inhibits angiogenesis in vitro and in vivo. Angiogenesis 14:187–197
Yang TH, Jia M, Meng J, Wu H, Mei QB (2006) Immunomodulatory activity of polysaccharide isolated from Angelica sinensis. Int J Biol Macromol 39:179–184
Jung SM, Schumacher HR, Kim H, Kim M, Lee SH, Pessler F (2007) Reduction of urate crystal-induced inflammation by root extracts from traditional oriental medicinal plants: elevation of prostaglandin D(2) levels. Arthritis Res Ther 9
Shibata S (2000) A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi-J Pharm Soc Jpn 120:849–862
Lucas (ed) (1976) Nature's medicines. Melvin Powers/Wilshire Book Company, No. Hollywood, pp 89–94
Huang KH (1959) Use of liquid extract of liquorice for pulmonary tuberculosis. Shandong Yi Kan 21:17–18
Das SK, Das V, Gulati AK, Singh VP (1989) Deglycyrrhizinated liquorice in aphthous ulcers. J Assoc Phys India 37:647
Krausse R, Bielenberg J, Blaschek W, Ullmann U (2004) In vitro anti-helicobacter pylori activity of extractum liquiritiae, glycyrrhizin and its metabolites. J Antimicrob Chemother 54:243–246
Kakegawa H, Matsumoto H, Satoh T (1992) Inhibitory effects of some natural-products on the activation of hyaluronidase and their antiallergic actions. Chem Pharm Bull 40:1439–1442
Chandrasekaran CV, Deepak HB, Thiyagarajan P et al (2011) Dual inhibitory effect of Glycyrrhiza glabra (GutGard) on COX and LOX products. Phytomedicine 18:278–284
Thiyagarajan P, Chandrasekaran CV, Deepak HB, Agarwal A (2011) Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology 19:235–241
Kim JK, Oh SM, Kwon HS, Oh YS, Lim SS, Shin HK (2006) Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem Bioph Res Co 345:1215–1223
Uto T, Morinaga O, Tanaka H, Shoyama Y (2012) Analysis of the synergistic effect of glycyrrhizin and other constituents in licorice extract on lipopolysaccharide-induced nitric oxide production using knock-out extract. Biochem Biophys Res Commun 417:473–478
Shin EM, Zhou HY, Guo LY et al (2008) Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis (Leguminosae) in LPS-induced RAW264.7 macrophages. Int Immunopharmacol 8:1524–1532
Kim YW, Zhao RJ, Park SJ et al (2008) Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappa B-dependent iNOS and proinflammatory cytokines production. Brit J Pharmacol 154:165–173
Tanaka A, Horiuchi M, Umano K, Shibamoto T (2008) Antioxidant and anti-inflammatory activities of water distillate and its dichloromethane extract from licorice root (Glycyrrhiza uralensis) and chemical composition of dichloromethane extract. J Sci Food Agr 88:1158–1165
Franceschelli S, Pesce M, Vinciguerra I et al (2011) Licocalchone-C extracted from Glycyrrhiza glabra inhibits lipopolysaccharide-interferon-gamma inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression. Molecules 16:5720–5734
Aly AM, Al-Alousi L, Salem HA (2005) Licorice: a possible anti-inflammatory and anti-ulcer drug. AAPS PharmSciTech 6:E74–E82
Kim KR, Jeong CK, Park KK et al (2010) Anti-inflammatory effects of licorice and roasted licorice extracts on tpa-induced acute inflammation and collagen-induced arthritis in mice. J Biomed Biotechnol. doi:10.1155/2010/709378
Gujral ML, Sareen K, Phukan DP, Amma MK (1961) Antiarthritic activity of glycyrrhizin in adrenalectomised rats. Indian J Med Sci 15:624–629
Zhang W, Lu C, Liu Z et al (2007) Therapeutic effect of combined triptolide and glycyrrhizin treatment on rats with collagen induced arthritis. Planta Med 73:336–340
Zhen QS, Ye X, Wei ZJ (1995) Recent progress in research on tripterygium - a male antifertility plant. Contraception 51:121–129
Lipsky PE, Tao XL (1997) A potential new treatment for rheumatoid arthritis: thunder god vine. Semin Arthritis Rheum 26:713–723
Wang B, Ma L, Tao X, Lipsky PE (2004) Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum 50:2995–3003
Tao XL, Lipsky PE (2000) The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum Dis Clin N Am 26: 29- +
Shao D, Dunlop WD, Lui EMK, Bernards MA (2008) Immunostimulatory and anti-inflammatory polysaccharides from Tripterygium wilfordii: comparison with organic extracts. Pharm Biol 46:8–15
Tao XL, Cai JJ, Lipsky PE (1995) The identity of immunosuppressive components of the ethyl-acetate extract and chloroform methanol extract (T2) of tripterygium-wilfordii hook-F. J Pharmacol Exp Ther 272:1305–1312
Mizel SB, Dayer JM, Krane SM, Mergenhagen SE (1981) Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (Interleukin-1). Proc Natl Acad Sci USA-Biol Sci 78:2474–2477
Maekawa K, Yoshikawa N, Du J et al (1999) The molecular mechanism of inhibition of interleukin-1 beta-induced cyclooxygenase-2 expression in human synovial cells by Tripterygium wilfordii Hook F extract. Inflamm Res 48:575–581
Tao XL, Schulze-Koops H, Ma L, Cai J, Mao YP, Lipsky PE (1998) Effects of Tripterygium wilfordii Hook F extracts on induction of cyclooxygenase 2 activity and prostaglandin E-2 production. Arthritis Rheum 41:130–138
Caterson B, Flannery CR, Hughes GE, Little CB (2000) Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol 19:333–344
Sylvester J, Liacini A, Li WQ, Dehnade F, Zafarullah M (2001) Tripterygium wilfordii Hook F extract suppresses proinflammatory cytokine-induced expression of matrix metalloproteinase genes in articular chondrocytes by inhibiting activating protein-1 and nuclear factor-kappa B activities. Mol Pharmacol 59:1196–1205
Yu KT, Nuss G, Boyce R et al (1994) Inhibition of Il-1 release from human monocytes and suppression of streptococcal cell-wall and adjuvant-induced arthritis in rats by an extract of tripterygium-wilfordii hook. Gen Pharmacol 25:1115–1122
Chou CT (1997) The antiinflammatory effect of an extract of Tripterygium wilfordii Hook F on adjuvant-induced paw oedema in rats and inflammatory mediators release. Phytother Res 11:152–154
Tao X, Ma L, Mao Y, Lipsky PE (1999) Suppression of carrageenan-induced inflammation in vivo by an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F. Inflamm Res 48:139–148
Gu WZ, Brandwein SR, Banerjee S (1992) Inhibition of type-ii collagen induced arthritis in mice by an immunosuppressive extract of tripterygium-wilfordii hook-F. J Rheumatol 19:682–688
Tao XL, Cush JJ, Garret M, Lipsky PE (2001) A phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii Hook F in rheumatoid arthritis. J Rheumatol 28:2160–2167
Tao XL, Younger J, Fan FZ, Wang B, Lipsky PE (2002) Benefit of an extract of Tripterygium wilfordii Hook F in patients with rheumatoid arthritis - a double-blind, placebo-controlled study. Arthritis Rheum 46:1735–1743
Krakauer T, Chen X, Howard OMZ, Young HA (2005) Triptolide attenuates endotoxin- and staphylococcal exotoxin-induced T-cell proliferation and production of cytokines and chemokines. Immunopharmacol Immunotoxicol 27:53–66
Lin N, Sato T, Ito A (2001) Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. f., suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheum 44:2193–2200
Liu QY, Chen TY, Chen GY et al (2006) Immunosuppressant triptolide inhibits dendritic cell-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-kappa B activation. Biochem Bioph Res Co 345:1122–1130
Wang Y, Jia L, Wu CY (2008) Triptolide inhibits the differentiation of Th17 cells and suppresses collagen-induced arthritis. Scand J Immunol 68:383–390
Dai SM, Nishioka K, Yudoh K (2004) Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis 63:1379–1386
Lu Y, Wang WJ, Leng JH, Cheng LF, Feng L, Yao HP (2008) Inhibitory effect of triptolide on interleukin-18 and its receptor in rheumatoid arthritis synovial fibroblasts. Inflamm Res 57:260–265
Liacini A, Sylvester J, Zafarullah M (2005) Triptolide suppresses proinflammatory cytokine-induced matrix metalloproteinase and aggrecanase-1 gene expression in chondrocytes. Biochem Biophys Res Commun 327:320–327
Zhou J, Xiao C, Zhao LH et al (2006) The effect of triptolide on CD4+ and CD8+ cells in Peyer's patch of SD rats with collagen induced arthritis. Int Immunopharmacol 6:198–203
Xiao C, Lu C, Zhao LH et al (2006) The effects of triptolide on enteric mucosal immune responses of DBA/1 mice with collagen-induced arthritis. Planta Med 72:1268–1272
Gu WZ, Brandwein SR (1998) Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol 20:389–400
Kim WU, Lee WK, Ryoo JW et al (2002) Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen - a novel treatment strategy for induction of oral tolerance. Arthritis Rheum 46:1109–1120
Wahl SM (1994) Transforming growth-factor-beta - the good, the bad, and the ugly. J Exp Med 180:1587–1590
Xiao C, Zhao LH, Liu ZL et al (2009) The effect of triptolide on CD4 + and CD8 + cells in the Peyer's patch of DA rats with collagen induced arthritis. Nat Prod Res 23:1699–1706
Lin N, Liu CF, Xiao C et al (2007) Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol 73:136–146
Bown D (ed) (1995) Encyclopaedia of herbs and their uses. Dorling Kindersley, London, pp 361–365
Chopra R, Nayar S, Chopra I (eds) (1986) Glossary of indian medicinal plants (Including the supplement). Council of Scientific and Industrial Research, New Delhi, pp 51–83
Diwan P, Karwande I, Singh A (1991) Anti-anxiety profile of mandukparni Centella asiatica Linn in animals. Fitoterapia 62:255–257
Boiteau P, Ratsimamanga A (1956) Asiaticoside extracted from Centella asiatica and its therapeutic uses in cicatrization of experimental and refractory wounds (leprosy, cutaneous tuberculosis and lupus). Therapie 11:125–149
Guo JS, Cheng CL, Koo MWL (2004) Inhibitory effects of Centella asiatica water extract and asiaticoside on inducible nitric oxide synthase during gastric ulcer healing in rats. Planta Medica 70:1150–1154
George M, Joseph L, Ramaswamy (2009) Anti-allergic, anti-pruritic, and anti-inflammatory activities of centella asiatica extracts. Afr J Tradit Complement Altern Med 6:554–559
Somchit M, Sulaiman M, Zuraini A et al (2004) Antinociceptive and antiinflammatory effects of Centella asiatica. Indian J Pharmacol 36:377–380
Hussin M, Abdul-Hamid A, Mohamad S, Saari N, Ismail M, Bejo MH (2007) Protective effect of Centella asiatica extract and powder on oxidative stress in rats. Food Chem 100:535–541
Hashim P, Sidek H, Helan MHM, Sabery A, Palanisamy UD, Ilham M (2011) Triterpene composition and bioactivities of centella asiatica. Molecules 16:1310–1322
Won JH, Shin JS, Park HJ et al (2010) Anti-inflammatory effects of madecassic acid via the suppression of NF-kappaB pathway in LPS-induced RAW 264.7 macrophage cells. Planta Med 76:251–257
Liu M, Dai Y, Yao XJ et al (2008) Anti-rheumatoid arthritic effect of madecassoside on type II collagen-induced arthritis in mice. Int Immunopharmacol 8:1561–1566
Li HZ, Gong X, Zhang L et al (2009) Madecassoside attenuates inflammatory response on collagen-induced arthritis in DBA/1 mice. Phytomedicine 16:538–546
Koch E (2001) Extracts from fruits of saw palmetto (Sabal serrulata) and roots of stinging nettle (Urtica dioica): Viable alternatives in the medical treatment of benign prostatic hyperplasia and associated lower urinary tracts symptoms. Planta Medica 67:489–500
Anonymous ed. (2003) Urticae folium/herba. In: European scientific cooperative on phytotherapy. Thieme, Stuttgart, New York: ESCOP Monographs. pp. 521–527 pp
Akbay P, Basaran AA, Undeger U, Basaran N (2003) In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother Res 17:34–37
Obertreis B, Ruttkowski T, Teucher T, Behnke B, Schmitz H (1996) Ex-vivo in-vitro inhibition of lipopolysaccharide stimulated tumor necrosis factor-a and interleukin-1 beta secretion in human whole blood by Extractum Urticae dioicae foliorum. Arzneim-Forsch/Drug Res 46:389–394
Harput US, Saracoglu I, Ogihara Y (2005) Stimulation of lymphocyte proliferation and inhibition of nitric oxide production by aqueous Urtica dioica extract. Phytother Res 19:346–348
Barnes PJ, Larin M (1997) Mechanisms of disease - nuclear factor-kappa b - a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071
Riehemann K, Behnke B, Schulze-Osthoff K (1999) Plant extracts from stinging nettle (Urtica dioica), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-kappa-B. FEBS Lett 442:89–94
Lanzavecchia A, Sallusto F (2001) Regulation of T cell immunity by dendritic cells. Cell 106:263–266
Broer J, Behnke B (2002) Immunosuppressant effect of IDS 30, a stinging nettle leaf extract, on myeloid dendritic cells in vitro. J Rheumatol 29:659–666
Schulze-Tanzil G, de Souza P, Behnke B, Klingelhoefer S, Scheid A, Shakibaei M (2002) Effects of the antirheumatic remedy Hox alpha - a new stinging nettle leaf extract - on matrix metalloproteinases in human chondrocytes in vitro. Histol Histopathol 17:477–485
Semerano L, Assier E, Boissier MC (2012) Anti-cytokine vaccination: a new biotherapy of autoimmunity. Autoimmun Rev 12: http://dx.doi.org/10.1016/j.autrev.2012.02.003
Nakken B, Munthe LA, Konttinen YT, Sandberg AK, Szekanecz A, Alex P, Szodoray P (2011) B cells and their targeting in rheumtoid arthritis - current concepts and future perspectives. Autoimmun Rev 11:28–34
Rosenblum H, Amital H (2011) Anti-TNF-therapy: safety aspects of taking the risk. Autoimmun Rev 10:563–568
Fillipini M, Bazzani C, Favalli EG et al (2010) Efficacy and safety of anti-tumour necrosis factor in elderly patients with rheumatoid arthrits: an observational study. Clin Rev Allergy Immunol 38:90–96
Guo KJ, Xu SF, Yin P, Wang W, Song XZ, Liu HF, Xu JQ, Zoccarato I (2011) Active components of common traditional Chinese medicine decoctions have antioxidant function. J Anima Sci 89:3107–3115
Guimaraes R, Barros L, Carvalho AM, Ferreira IC (2011) Infusions and decoctions of mixed herbs used in folk medicine: synergism in antioxidant potential. Phytother Res 25:1209–1214
Hayashi K, Shimura K, Makino T, Mizukami H (2010) Comparison of the contents of kampo decoctions containing ephedra herb when prepared simply or by re-bioling according to the traditional theory. J Nat Med 54:70–74
Acknowledgements
This project was supported in part by grants from Prof Francis SK Lau Research Fund and PuraPharm International awarded to Prof. A Lau.
Author information
Authors and Affiliations
Corresponding author
Additional information
Cindy L. H. Yang and Terry C. T. Or contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, C.L.H., Or, T.C.T., Ho, M.H.K. et al. Scientific Basis of Botanical Medicine as Alternative Remedies for Rheumatoid Arthritis. Clinic Rev Allerg Immunol 44, 284–300 (2013). https://doi.org/10.1007/s12016-012-8329-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-012-8329-8